November 2025 Newsletter

Volume 31, Issue 4
Table of Contents:
-
Perspectives: Tension Between Collective and Individual Benefit
-
Donor and Recipient Health Services Working Committee Update
-
ACT Corner: Leadership Transition at CIBMTR ACT: Passing the Baton
Perspectives: Tension Between Collective and Individual Benefit
By Sumithira Vasu, MBBS, MD
"We don't want to change. Every change is a menace to stability. That's another reason why we're so chary of applying new inventions."
-Aldous Huxley, Brave New World
"To be or not to be."
-William Shakespeare, Hamlet
In February 2025, we were fresh off from a great Tandem Meetings in Hawaii, reminiscing about the role of the institutional transplant ecosystem, our national and international community that engages robustly in data sharing, publicly reported outcomes, and research.
This November, CIBMTR will host the Center Outcomes Forum (led by Douglas Rizzo, MD, MS) to discuss results from several working groups to refine and enhance the data collection and reporting of outcomes, which will impact the entire transplant community. Due to CIBMTR’s mandate for public reporting, rigorous, frequent, and scientific monitoring of quality of care and outcomes is an integral part of every transplant center.
The AML field has rapidly evolved in terms of both molecular characterization and therapies that can now be given in the outpatient setting. In addition to complete remission, today's therapies often result in a morphologic leukemia-free state, or remission with persistent genetic abnormalities. Specifically, TP53 mutated AML is the subject of intense debate within the transplant community and among referring physicians. Literature shows that one-year overall survival after allogeneic transplant for TP53 AML can be as low as 15 to 23%. Some transplant centers have chosen not to transplant these patients at all, while others offer a relapse mitigation trial. Transplant physicians spend an extraordinary amount of time explaining risks and benefits, and when a patient states, “Even if it’s a 20% chance of survival, I will take the risk,” it is a challenging situation to navigate. While data show that patient selection is not affected in the three years following a “worse than expected” designation on the center-specific survival analysis,1 data also show that transplant volumes do decline when centers perform worse than expected.2 Widespread adaptation of new therapies addressing primary induction failure have not come to pass, likely an unintended consequence of centers trying not to fare poorly.3 Does the current center-specific survival analysis statistical model properly adjust for modern AML predictive covariates (also an important question with AML in CR1)? Is the statistical model performing well when transplant volumes are small?
The transplant clinician is faced with the task of making a transplant candidacy decision based on whether it is for the individual benefit of the patient vs. the collective benefit for the institution that can continue to provide a lifesaving therapy for patients. Risk is viewed differently from a patient versus physician perspective, which explains why “right to try” acts have been passed in Congress.
So, how do we address this question when we see patients whose disease risk makes it unlikely for them to survive to 1 year? As a community, do we address this challenge head-on, by designing trials that attempt to offer transplant to high-risk patients to expand access to transplant? Would a specific dispensation allowing lower risk estimates incentivize centers to do such transplants? To quote our 2025 CIBMTR Mortimer M. Bortin lecturer, Sergio Giralt, MD, “The best way to convert an MRD positive patient into MRD negative is through a transplant!” Is it time to include a different score when modeling expected outcomes for treatment within a clinical trial for patients with, say, less than 40% one-year survival expectation?
CIBMTR has been modifying the model over the last few decades to include known prognostic factors so that the 2025 model will include new risk factors to adjust for Europena LeukemiaNet (ELN) risk stratification, including TP53 mutations. Additional poor prognostic factors, such as MRD positivity pre-transplant, that negatively impact survival could help further refine the models and studying their impact on transplant practice would be imperative.
We can certainly lean on the argument that there is always a tradeoff between collective versus individual benefits. Vaccines are a good example highlighting the collective good over rare risks. These questions don’t have easy answers, and yet we, as a community, have to wrestle with them, engage in constructive, solution-oriented dialogue to make transplant accessible and effective for all patients, not just a subset of patients. There is a CIBMTR session planned for the 2026 Tandem Meetings explaining the changes to the model. Let’s continue this dialogue at the Tandem Meetings, with a dedicated CIBMTR session discussing future research ideas about the impact of public reporting of outcomes on Wednesday, February 4, 2026, at 10:30 AM – 12 PM in Salt Lake City.
To paraphrase Shakespeare, to transplant or not to transplant, that is the question.
References
- Strouse C, Juckett M, Logan BR, et al. Impact of publicly reported outcomes on patient selection for hematopoietic cell transplantation. JCO Oncology Practice. 2025 Oct 2: OP2500115. doi: 10.1200/OP-25-00115. PMC12494149. [Epub ahead of print].
- Sharma A, Logan B, Estrada-Merly N, et al. Impact of public reporting of center-specific survival analysis scores on patient volumes at hematopoietic cell transplant centers. Transplantation and Cellular Therapy. 2023 Aug 1; 29(8):523-528. doi: 10.1016/j.jtct.2023.05.013. Epub 2023 May 21. PMID: 37220838.
- Gyurkocza B, Nath R, Seropian S, et al. Randomized Phase III SIERRA trial of 131I-apamistamab before allogeneic hematopoietic cell transplantation versus conventional care for relapsed/refractory AML. Journal of Clinical Oncology. 2025 Jan 10; 43(2):201-213. doi: 10.1200/JCO.23.02018. Epub 2024 Sep 19. PMC11709001.
New Immunobiology Working Committee Co-Scientific Director
CIBMTR is proud to introduce Rohtesh Mehta, MD, MPH, MS, as a new co-Scientific Director of the Immunobiology Working Committee.
Dr. Mehta is an Associate Professor in Stem Cell Transplantation & Cellular Therapy at the University of Texas MD Anderson Cancer Center. He received his MD in 2002, MPH in epidemiology in 2006, and MS in clinical research in 2011. He completed his residency in internal medicine in 2009 as well as fellowships in palliative care and hematology-oncology at the University of Pittsburgh Medical Center and in stem cell transplantation at the University of Texas MD Anderson Cancer Center.
During his fellowships, Dr. Mehta focused on generating cord-blood derived autologous T-cells for AML treatment and investigated NK cell biology for stem cell transplantation. This research culminated in leading a prospective clinical trial evaluating adoptive NK cell therapy in multiple myeloma patients undergoing autologous transplantation.
Dr. Mehta established and continues to chair the Donor Integrated Selection Consortium (DISCo), a collaborative involving HCT centers across the US, dedicated to the development and implementation of evidence-based contemporary donor selection strategies and comprehensive transplant approaches. Through the years, he has initiated and collaborated on multiple CIBMTR studies investigating the role of donor-related HLA and non-HLA factors. His involvement in practice-changing research includes insights into the efficacy and safety of alternative donor HCT and factors for optimizing donor selection for best patient outcomes.
His publication record includes more than 145 peer-reviewed manuscripts, including corresponding and lead author studies in immunobiology and HCT and recent studies employing machine learning approaches. International organizations recognize Dr. Mehta as a leader in the field, as evidenced by invitations to speak and chair plenary sessions at transplantation, histocompatibility, and cellular therapy conferences worldwide. He is also dedicated to mentoring and training future leaders in HCT as shown by the multiple completed and ongoing studies with junior faculty and trainees.
CIBMTR selected Dr. Mehta through an extensive interview process, with special thanks to leaders and administrators at NMDP and the Medical College of Wisconsin.
Please join CIBMTR in welcoming him as a CIBMTR Scientific Director.
Donor and Recipient Health Services Working Committee Update
After careful consideration, we have made the difficult decision to discontinue the Donor and Recipient Health Services Working Committee. This decision is driven by challenges limiting the committee’s intended goal in delivering high-level impact work. As we move forward, we will discontinue all current Donor and Recipient Health Services Working Committee studies and will no longer nominate individuals to the committee.
In alignment with CIBMTR’s strategic pillar, Accelerate Practice-Changing Research, the Implementation Science Program now includes health services research (HSR). We believe this integration more poignantly reflects HSR’s impact in driving the rapid adoption of research into practice. HSR plays a critical role in understanding the current state within the implementation science cycle and continues to define systems-level change through advocacy and policy. Investigators are encouraged to submit PRO-based proposals through any working committee, as another way to incorporate HSR-related topics.
We want to reassure the community that CIBMTR will continue to prioritize donor safety. Donor safety remains central to the mission of NMDP, and NMDP actively evaluates clinical events in real time. When donor safety issues arise, NMDP medical staff review them internally, and the Donor Safety Monitoring Board conducts external reviews to ensure comprehensive oversight.
Investigators can submit funding proposals relevant to donor safety and HSR directly through CIBMTR’s website. The working committees will consider and evaluate these proposals through the established process.
Non-Malignant Diseases Working Committee
Committee Leadership
Co-Chairs:
- Ashish Gupta, University of Minnesota Blood and Marrow Transplant Program – Pediatrics, Minneapolis, MN
- Carmem Bonfim, Duke University, Durham, NC
- Kasiani Myers, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
Scientific Director:
- Larisa Broglie, CIBMTR MCW, Milwaukee, WI
Statistical Director:
- Soyoung Kim, CIBMTR MCW, Milwaukee, WI
Statistician:
- Yongzi Yu, CIBMTR MCW, Milwaukee, WI
CIBMTR Page Scholar:
- Jane Koo, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
The Non-Malignant Diseases Working Committee leads clinical research on early and late outcomes following allogeneic HCT for non-cancer indications. Because many of the diseases are rare or ultra-rare and involve small patient populations, the committee promotes multi-institutional collaborative studies to gather the most comprehensive data on HCT outcomes. The committee also fosters international collaboration to expand cohort sizes and ensure findings are generalizable to the broader non-malignant HCT population.
The committee has collaborated with Eurocord and individual highly specialized centers to report on transplant-related topics, advance scientific knowledge, and guide clinical practice in this area. This committee’s research focuses on a wide range of disorders, broadly classified under the following categories: Marrow failure (acquired and inherited), hemoglobinopathies, metabolic disorders, immune deficiency / dysregulation disorders, and autoimmune diseases.

The committee manages a wide variety of diseases, which share several important characteristics. Most patients with non-malignant diseases who undergo transplant-based therapies are young (including infants), so they often experience different toxicities than adults. Because of their age, the potential to positively affect their lives through successful treatment is especially significant.
Experts in the field lead the Non-Malignant Diseases Working Committee, bringing unique perspectives to help develop high-impact studies. Carmem Bonfim, MD, PhD, contributes her expertise in Fanconi anemia and aplastic anemia. Ashish Gupta, MBBS, MPH, focuses on transplant and gene therapies for hemoglobinopathies and inherited metabolic diseases. Kasiani Myers, MD, brings her experience in inherited bone marrow failure syndromes and her work at Cincinnati Children’s Hospital Medical Center to the committee. This summer we also welcomed Jane Koo, MD, MA, BA, to the leadership team as our Page Scholar. Jane will collaborate with committee leaders to learn the inner workings of CIBMTR and oversee ongoing studies within the working committee. Yongzi Yu, MS, and Soyoung Kim, PhD, lead statistical efforts, and Larisa Broglie, MD, MS, serves as the committee’s scientific director.
The Working Committee has a very active and broad research portfolio with 10 ongoing studies, in various stages:
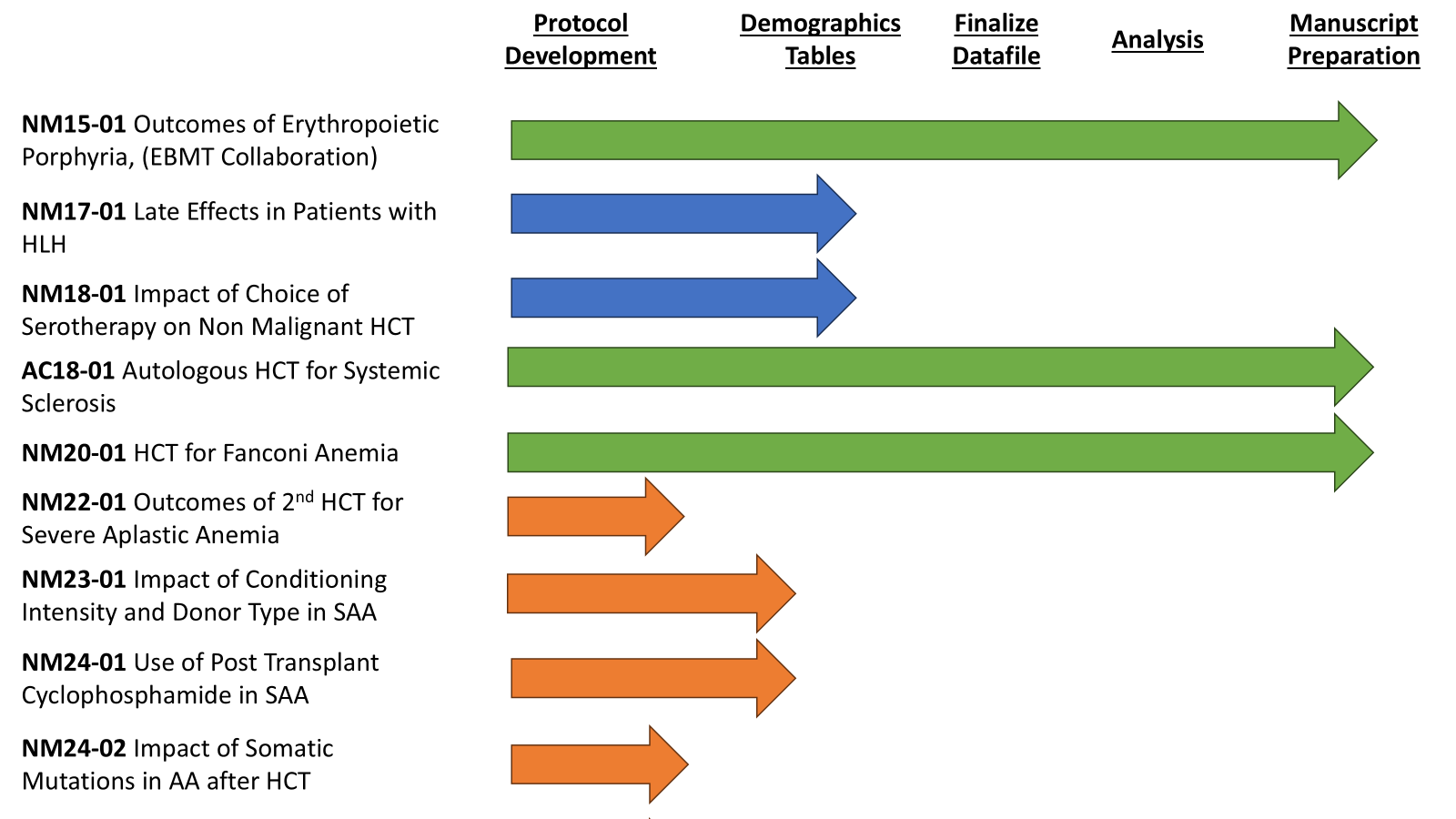
Investigators from diverse backgrounds actively contribute to the Non-Malignant Diseases Working Committee, bringing valuable experience in transplantation for benign, often rare disorders. The wide range of diseases, along with shared transplant goals, creates strong opportunities for scientific synergy and innovation. A full list of the committee’s studies, including recent publications, is available on the Non-Malignant Diseases Working Committee studies webpage. Each year at the Tandem Meetings, committee members select 1-2 new proposals to advance to protocol development. Members score and prioritize proposals based on interest and potential impact. We invite investigators to participate actively by submitting new proposals and providing input throughout the protocol, analysis, and manuscript phases. The committee thrives on engagement from the scientific community, and we especially encourage junior investigators to provide input. To learn more about our committee or to discuss ideas and projects, please reach out to a committee co-chair or the scientific director.
Plasma Cell Disorders Working Committee
 |
 |
 |
| Heather Landau | Taiga Nishihori | Yvonne Efebera |
 |
 |
|
| Othman Salim Akhtar | Tao Wang | |
 |
||
| Temitope Oloyede |
Committee Leadership
Co-Chairs:
- Heather Landau, Memorial Sloan Kettering Cancer Center, New York, NY
- Taiga Nishihori, Moffitt Cancer Center, Tampa, FL
- Yvonne Efebera, OhioHealth, Columbus, OH
Scientific Director:
- Othman Akhtar, MD, CIBMTR MCW, Milwaukee, WI
Statistical Director:
- Tao Wang, CIBMTR MCW, Milwaukee, WI
Statistician:
- Temitope Oloyede, CIBMTR MCW, Milwaukee, WI
Multiple myeloma remains the most common indication for autologous HCT in the US. The development of B-cell maturation antigen (BCMA)-directed CAR T-cell therapy has dramatically changed how clinicians treat multiple myeloma. The Plasma Cell Disorders Working Committee actively collaborates with investigators worldwide to define best practices for using transplantation and CAR-T therapy—not only for multiple myeloma but also other plasma cell disorders. These include light chain amyloidosis, Waldenstrom macroglobulinemia, monoclonal gammopathy of renal or clinical significance, POEMS syndrome, and plasma cell leukemia.
We are actively leading several projects that explore key aspects of plasma cell disorders and their treatment. Our current studies examine risk factors and characteristics of second primary malignancies following autologous HCT for multiple myeloma, outcomes of autologous HCT for light chain deposition disease; prolonged cytopenia following anti-BCMA CAR-T therapy for multiple myeloma, the safety and efficacy of ciltacabtagene in patients with relapsed or refractory multiple myeloma, and real-world comparisons of anti-BCMA CAR-T therapies in relapsed or refractory multiple myeloma. Last year, we accepted two additional studies: One investigates predictors of early relapse and durable remissions in patients with multiple myeloma treated with BCMA-targeted CAR-T therapy, and the other evaluates outcomes of out-of-specification BCMA-directed CAR-T therapies in heavily pretreated patients with relapsed / refractory multiple myeloma.
Since our last newsletter article in 2024, the committee published 3 peer-reviewed journal articles and delivered 2 presentations at the International Myeloma Society Annual Meeting.
For the 2025 Tandem Meetings, the committee received 40 proposals. After combining multiple submissions, investigators presented 19 proposals at the meetings. From those, we accepted 3 proposals—representing a total of 5 combined submissions—as new working committee studies.
The Plasma Cell Disorders Working Committee actively seeks innovative and impactful study ideas, and we encourage junior investigators and those new to HCT and outcomes research to get involved. We believe that early engagement fosters long-term participation and helps build a strong pipeline of future leaders in the field. Our committee aims to maintain a positive feedback loop that drives greater national and international collaboration in transplant and CAR-T research. Through these efforts, we strive to accelerate the discovery of cures for patients affected by plasma cell disorders.
The Plasma Cell Disorders Working Committee invites all investigators interested in these diseases to propose a study. Investigators can find information on how to submit a proposal on CIBMTR's Propose a Working Committee Study webpage.
To learn more or share your research ideas and proposals, reach out to a member of the working committee leadership team. We encourage our more than 600 committee members to participate actively. We look forward to seeing you next February at the 2026 Tandem Meetings in Salt Lake City, UT!
A Note of Appreciation:
 Marcelo Pasquini, MD, MS, former Scientific Director of the Plasma Cell Disorders Working Committee, recently relocated to Cleveland, OH, to serve as the Transplant and Cellular Therapy Medical Director for Cleveland Clinic. The committee wishes Dr. Pasquini the best in his new role:
Marcelo Pasquini, MD, MS, former Scientific Director of the Plasma Cell Disorders Working Committee, recently relocated to Cleveland, OH, to serve as the Transplant and Cellular Therapy Medical Director for Cleveland Clinic. The committee wishes Dr. Pasquini the best in his new role:
“We want to thank Dr. Marcelo Pasquini for his invaluable contributions to our working committee as Scientific Director. His deep knowledge, diligence, attention to detail, and exemplary leadership is greatly appreciated. As we send heart-felt congratulations, we wish him the very best in the next phase of his career.”
CIBMTR Page Scholars
Over the coming months, we will continue to highlight each of the Page Scholars to further introduce them to our CIBMTR community. Each participant will share who they are, what their goals are, and why they’re interested in the Page Scholar Program.
 |
Xia Bi, MD, MS – Leukemia Working Committee I am an Assistant Professor in the Department of Bone Marrow Transplant and Cellular Therapy at Thomas Jefferson University. My clinical and research interests focus on investigator-initiated trials aimed at improving outcomes after allogeneic HCT, with particular emphasis on incorporating novel GVHD prophylaxis strategies for patients with high-risk hematologic malignancies. Through participation in CIBMTR’s Page Scholar Program, I hope to gain deeper insight into CIBMTR’s processes and contribute to the advancement of study design, with the ultimate goal of driving meaningful improvements in transplantation outcomes. “Dr. Bi has been extremely involved with the Leukemia Working Committee. She is currently leading ongoing protocols and has submitted novel concepts as new proposals for the 2026 Tandem Meetings!” |
|
 |
Jane Koo, MD – Non-Malignant Working Committee I am a pediatric bone marrow transplanter at Cincinnati Children's Hospital Medical Center with a specialization in non-malignant diseases, more specifically inherited bone marrow failure syndromes (IBMFS) and idiopathic aplastic anemia. Currently, I conduct both clinical and translational research focusing on IBMFS and complications following HCT, including acute and chronic lung injury. I am honored to serve as a Page Scholar and am looking forward to learning the inner workings of CIBMTR, including performing critical review of incoming research proposals, understanding the process of statistical analysis, and comprehending how to leverage large datasets to answer impactful scientific questions to drive practice changes within the HCT field. “It has been a pleasure to have Dr. Koo as part of the Non-Malignant Working Committee. She brings her expertise in marrow failures and an enthusiasm in engaging with the ongoing studies and learning about CIBMTR processes. We are excited for her to take leadership roles on upcoming studies.” |
|
 |
Takuto Takahashi, MD, PhD – Pediatric Cancer Working Committee I am an Assistant Professor in the Stem Cell Transplantation Program at Dana-Farber / Boston Children’s Cancer and Blood Disorders Center. As an aspiring clinical investigator, my interests include pediatric malignancies, long-term outcomes of childhood cancer and HCT, and clinical pharmacology. I recently joined the Pediatric Cancer Working Committee as a Page Scholar and see this program as an invaluable opportunity to expand my understanding of CIBMTR resources, strengthen my ability to design impactful studies, and develop the skills necessary to optimize collaborative research. I am eager to learn from experienced leaders in the field and to contribute meaningfully to advancing CIBMTR’s mission. “Dr. Takahashi has come to the Pediatric Cancer Working Committee with a lot of enthusiasm and is ready to learn and understand CIBMTR processes. I look forward to him taking on leadership roles within the committee and guiding us in our ongoing studies.” |
Register Now for the 2026 Tandem Meetings!
By Alicia Halfmann and Maira Brey
Registration is now open for the 2026 Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT® and CIBMTR®, taking place February 4-7, 2026, in Salt Lake City, UT.
The Tandem Meetings is a multidisciplinary event highlighting the latest research and breakthroughs in the evolving field of cellular therapy, including HCT, CAR-T, and gene therapy.
Register today to join us as a either an in-person attendee or digital access attendee to connect, learn, and advance your expertise. All attendees will receive access to on-demand session recordings and continuing education credits for up to 30 days post-event.
Book your hotel within the Tandem Meetings housing block after registering. Click here for more information.
Visit the Tandem Meetings Website for additional details and the program agenda.
Support the meetings and promote your organization through exhibits, sponsorships, and branding opportunities. Learn more and reserve your spot via our Exhibitor and Supporter pages.
We look forward to seeing you in Salt Lake City!
Follow ASTCT, CIBMTR, and #Tandem26 on social media for updates.
Barriers to Transplant: Caregiver Requirements
CIBMTR's Implementation Science Team, partnering with numerous collaborators, is actively working to identify and address barriers to transplant caused by strict caregiver requirements. These 24/7 caregiving demands can limit access to allogeneic HCT. To tackle this issue, the team launched Reimagining Caregiving Together, Engagement to Address Caregiver Requirement Barriers—a year-long initiative featuring two workshops sponsored by NMDP and funded by the Patient Centered Outcomes Research Institute (PCORI). These workshops aimed to co-create a patient-centered comparative effectiveness research agenda that will generate evidence on alternative post-allogeneic HCT care models and expand access to live-saving treatment.
In October 2024 and May 2025, the Implementation Science team hosted two dynamic workshops that brough together the diverse HCT community. These events actively engaged patients, caregivers, community and transplant center physicians, advanced practice providers, nurses, coordinators, social workers, payers, advocacy groups, government affairs professionals, industry representatives, pharmacists, implementation scientists, and researchers.
The group developed a focused research agenda targeting five key areas:
- Raising awareness of caregiver-related barriers and building support for alternative care models
- Clarifying the current landscape of caregiver requirements in allogeneic HCT
- Designing patient-centered care models that reflect diverse needs
- Driving change through policy reform and advocacy
- Collaborating with communities to co-create solutions
This agenda empowers action and fosters collaboration across and beyond the HCT community to conduct patient-centered comparative effectiveness research. The ultimate goal is to expand access for patients without caregivers and reduce the burdens placed on those who provide care.
To learn more or share your ideas, please reach out at caregiverstudy@nmdp.org.
Improving Access to Transplant: BMT CTN 1702 Donor Search Study Shows Comparable Outcomes with Alternative Donors
A recent multicenter study, BMT CTN 1702, demonstrates that patients who are unlikely to find a matched unrelated donor (MUD) for HCT can achieve comparable survival outcomes using alternative donors.
The study enrolled 1,751 evaluable participants across 47 centers, categorizing them by their likelihood of finding a MUD: Very likely (>90%), less likely, and very unlikely (<10%). Researchers prioritized patients in the very unlikely group for alternative donor options, including haploidentical related donors, MMUD, or umbilical cord blood.
Key findings from the study reveal:
- Two-year survival rates showed no significant difference between patients in the very likely and very unlikely groups.
- Of the 1,179 patients who underwent transplantation, 94% of the very likely group received a MUD, compared to 38% of the less likely group and 9% of the very unlikely group.
- Multivariate analyses confirmed no significant differences across groups in relapse rates, treatment-related mortality, disease-free survival, or incidence of GVHD.
These findings validate the use of a donor search prognosis score to guide early decision-making and accelerate access to transplant for patients with limited MUD options.
CIBMTR CRO Services Team Contributions
As part of the BMT CTN Data and Coordinating Center, the CIBMTR CRO Services team played a critical role in driving the study forward through every phase:
- Protocol Development: Collaborated closely with investigators and stakeholders to design a protocol grounded in real-world clinical practice.
- Study Management: Directed study operations across 47 participating sites, managing regulatory processes and tracking timelines to keep the study on course.
- Site Support: Provided hands-on guidance and troubleshooting to ensure consistent implementation of study procedures, including accurate data entry, timely query resolution, and ongoing communication with site staff.
- Arm Assignments: Partnered with NMDP immunogenetic specialists to assign participants to study arms using a donor search algorithm.
- PRO Collection: Led the collection of PRO data to support secondary study objectives and enrich the evidence base.
The CIBMTR CRO Services team ensured consistent operations across a large and diverse network of transplant centers, playing a pivotal role in the study’s success. Coordinating protocol implementation, streamlining data workflows, and actively engaging sites, the team upheld the integrity and completeness of the study dataset. This strong operational foundation enabled timely analysis and directly contributed to evidence that informs donor selection strategies and expands transplant access for patients with limited donor options.
ACT Corner
By Marcelo Pasquini, MD, MS, and Tiffany Hunt, MS
Update on CAR-T Toxicities Form
In 2025, the primary goal for CIBMTR’s ACT initiatives was to prioritize improving the capture of CAR-T toxicities. CIBMTR’s Cellular Therapy Essential Data forms, now in their ninth revision, have continuously evolved to keep pace with the rapidly advancing field of cellular immunotherapy. As new CAR-T therapies emerged and novel toxicities were identified and graded, the team updated the forms through successive revisions to ensure they accurately reflect current clinical realities and support high-quality data collection.
The team identified several gaps, including unclear questions and fields that were rarely completed. For instance, early versions of the forms included organ toxicities based on initial cytokine release syndrome (CRS) grading criteria. However, sections addressing grade 3 and 4 organ toxicities showed high rates of missing data, highlighting the challenges centers faced in accurately capturing these events. In contrast, CRS data were consistently captured, with rates comparable to those seen in pivotal CAR-T trials. This discrepancy underscores the registry’s strength in collecting well-defined, easily extractable outcomes from medical records, while also revealing its limitations in detecting broad adverse events, particularly severe organ toxicities (grades 3 and 4).
This insight prompted a focused review aimed at streamlining the collection of CAR-T toxicities.
Another key driver of the review was the reconvening of the ASTCT CAR T-Cell Toxicity Group in Fall 2024. This group revisited its original recommendations, which shaped the development of CRS grading criteria and the classification of immune effector cell-associated neurotoxicity syndrome. Through this renewed effort, the group clarified and refined definitions for both classic and emerging CAR-T toxicities.
In response, CIBMTR’s ACT team applied updated toxicity grading principles to revamp the CAR-T toxicity capture process in the tenth revision of the Cellular Therapy Essential Data suite of forms.
To advance this effort, CIBMTR’s Forms Development and ACT teams assembled a panel of data managers and cell therapy experts to help shape the revisions. Their collaborative approach focused on streamlining data collection and aligning form content with information routinely documented in medical records.
CIBMTR’s ACT team gratefully acknowledges the contributions of the following committee members:
- Sairah Ahmed, MD, MD Anderson Cancer Center
- Shaun DeJarnette, University of Kansas
- Christine Gibson, Roger Williams Medical Center
- Megan Herr, PhD, Roswell Park Cancer Institute
- Annie Im, MD, University of Pittsburgh Medical Center
- Brandon Loudon, Children’s Hospital of Philadelphia
- Megan Miller, Roswell Park Cancer Institute
- Kevin McNerney, MD, Ann & Robert H. Lurie Children's Hospital of Chicago
- Connie Nelson, University of California, San Diego Medical Center
- Amanda Olson, MD, MD Anderson Cancer Center
- Seth Rotz, MD, Cleveland Clinic
- Susan Staples, University of Kansas
Summary of Key Revisions:
- CRS: Added field for date of initiation of treatment, consolidated CRS-specific laboratory results
- Immune Effector Cell-associated HLH Syndrome (IEC-HS): Updated to align with current identification (diagnostic and laboratory data), grading, and treatment practices
- Neurotoxicities: Revised in accordance with ASTCT recommendations; this section now includes distinct syndromes:
- Classic ICANS: Questions necessary for grading and therapy
- Parkinsonism: Signs and symptoms that are associated with Parkinsonism
- Cranial nerve palsy
- Tumor inflammation-associated neurotoxicity (TIAN)
- Guillain-Barré syndrome
- Other neurotoxicities
- Questions now focus on essential grading elements and include specific treatment options.
- Hypogammaglobulinemia: Clarified intent of therapy and added field for infusion start date
- Grade 3 & 4 Organ Toxicities: Removed these questions due to low data capture rates and limited utility
- CIBMTR has scheduled the new forms for release in January 2026.
Leadership Transition at CIBMTR ACT: Passing the Baton
 |
 |
We are pleased to announce a leadership transition within the CIBMTR ACT initiative. Marcelo C. Pasquini, MD, MS, Senior Scientific Director, passed the baton to Amy Moskop MD, MS, assuming leadership of the ACT initiative. Dr. Pasquini now leads the Transplant and Cellular Therapy Program at Cleveland Clinic, Taussig Cancer Center.
Over the past five years, Drs. Pasquini and Moskop have worked closely to advance the development and application of CIBMTR’s cellular therapy database. Under Dr. Pasquini’s mentorship, Dr. Moskop led the previous Cellular Therapy Working Committee, in which she spearheaded studies focused on cellular therapy toxicities.
Dr. Moskop and Pasquini have worked together in the development and conduct of prospective PASS for CAR-T and gene therapy products with industry partners. These efforts have enabled long-term patient follow-up through CIBMTR’s infrastructure, supporting regulatory requirements for initial approval and label expansion of genetically modified cellular therapy products.
As incoming ACT lead, Dr. Moskop will continue to build on this foundation by expanding the cellular therapy research portfolio and streamlining data collection processes to reduce burden on contributing centers.
We thank Dr. Pasquini for his leadership and look forward to the continued growth of the ACT initiative under Dr. Moskop’s guidance.
CAR-T and Fertility Survey
Health care providers, have you ever wondered about the effects CAR-T may have on your patients’ fertility?
Help The Leukemia & Lymphoma Society in partnership with Citizen Health, Moffitt Cancer Center, and University of Florida, gather critical information to determine if CAR-T affect fertility by referring patients to this survey. Survey results will help researchers understand if CAR-T affects fertility and support informed strategies for future patients.
Who can participate?
- Adults over the age of 18
- Received CAR-T therapy as a result of a blood cancer diagnosis
Click here to view the flier and learn more about this survey.
Publicly Available Datasets
 In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, CIBMTR makes the final datasets from published studies publicly available on CIBMTR’s Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, CIBMTR makes the final datasets from published studies publicly available on CIBMTR’s Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
While providing these data, CIBMTR is committed to safeguarding the privacy of participants and protecting confidential and proprietary data. Upon accessing the datasets page on CIBMTR’s public website, the viewer is notified that the dataset was collected by CIBMTR, and CIBMTR’s supporters are listed. The webpage also clearly notes the terms and conditions of dataset usage.
NEW datasets are now available online.
Share Research in Plain Language
By Jennifer Motl
These new plain-language summaries of CIBMTR research may help your patients:
|
|
Patients want info about quality of life after transplant: Patients can help researchers design studies; read more: |
|
|
Transplant centers should help patients find care after BMT: Requiring a 24-hour caregiver blocks some patients from getting BMT; read more: |
 |
For patients unlikely to have a fully matched donor, their team should quickly switch to searching for alternate (mismatched) donors; read more: |
Find more summaries at:
Our Supporters
CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; 75R60222C00008, 75R60222C00009, and 75R60222C00011 from the Health Resources and Services Administration (HRSA); and N00014-25-1-2146 from the Office of Naval Research.
Additional federal support is provided by OT3HL147741, P01CA111412, R01CA100019, R01CA218285, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01FD008187, R01HL171117, U01AI069197, U01AI184132, U24HL157560, and UG1HL174426.
Support is also provided by Australian Bone Marrow Donor Registry; Boston Children’s Hospital; Fred Hutchinson Cancer Center; Gateway for Cancer Research, Inc.; Jeff Gordon Children’s Foundation; Medical College of Wisconsin; NMDP; Patient Center Outcomes Research Institute; PBMTF; St. Baldricks’s Foundation; Stanford University; Stichting European Myeloma Network (EMN); and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune LLC; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc.; Amgen, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; Autolus Limited; Beam; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, inc.; Blueprint Medicines; Bristol Myers Squibb Co.; CareDx Inc.; Caribou Biosciences, Inc.; CSL Behring; CytoSen Therapeutics, Inc.; DKMS; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gift of Life Biologics; Gift of Life Marrow Registry; HistoGenetics; ImmunoFree; In8bio, Inc.; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Japan Hematopoietic Cell Transplantation Data Center; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin International plc; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Medexus; Merck & Co.; Mesoblast, Inc.; Millennium, the Takeda Oncology Co.; Miller Pharmacal Group, Inc.; Miltenyi Biomedicine; Miltenyi Biotec, Inc.; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc.; OriGen BioMedical; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pierre Fabre Pharmaceuticals; PPD Development, LP; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc.; Sobi, Inc.; Sociedade Brasileira de Terapia Celular e Transplante de Medula Óssea (SBTMO); Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Tscan Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.




