August 2025 Newsletter

Volume 31, Issue 3
Table of Contents:
-
Perspectives: It Takes a Village to Launch a Moonshot: The New Era of Cell and Gene Therapy
-
Najla El Jurdi, MD, to Serve as Scientific Director of Graft-versus-Host Disease Working Committee
-
Spotlight on CIBMTR at ISOQOL 2025: Advancing Patient-Centered Research in Cellular Therapy
Perspectives: It Takes a Village to Launch a Moonshot: The New Era of Cell and Gene Therapy
By Sumithira Vasu, MBBS, MD
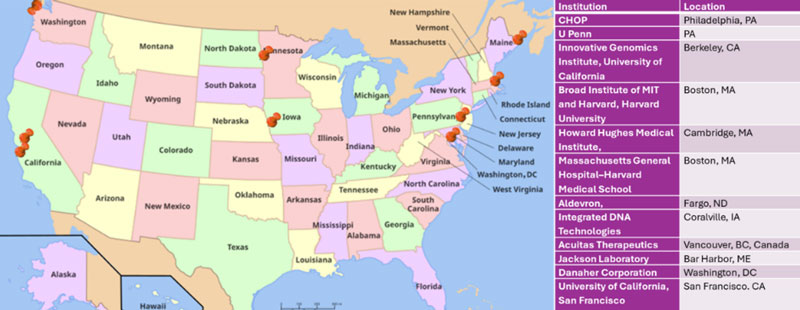
List of participating institutions to deliver the bespoke n=1 CRISPR based corrective therapeutic for Baby KJ.
Gene therapy has entered a transformative era, becoming increasingly personalized, precise, and accessible — a shift made possible through unprecedented global collaboration among researchers. Two recent landmark studies published in the New England Journal of Medicine — one led by Kiran Musunuru, et al. on base editing for a rare metabolic disorder1 and another by Alok Srivastava, et al. on lentiviral gene therapy for hemophilia A2 — vividly illustrate how international, interdisciplinary cooperation is driving innovation and expanding access to cutting-edge treatments in both resource-rich and resource-constrained settings.
Musunuru’s study exemplifies a paradigm-shifting approach to personalized, corrective CRISPR-based therapeutics, highlighting the rapid development of a bespoke gene-editing therapy that a multidisciplinary team tailored for a single patient, “Baby KJ,” who has a rare, life-threatening, inherited metabolic deficiency. Within 6 months of diagnosis, the team developed, tested, and delivered a lipid nanoparticle-based adenine base editor that precisely corrected the baby’s unique mutation. This example shows the potential, promise, and complexity of delivering gene therapy products.
This is a quintessential example of what is possible when people join forces – a successful N-of-1 approach requiring extensive coordination between academic institutions (Children’s Hospital of Philadelphia, University of Pennsylvania, University of California San Francisco, Harvard University), regulatory agencies, preclinical model developers, industry providing critical reagents, and clinical experts spanning hepatology, genetics and immunology. Importantly, this precedent establishes a scalable platform where developers reuse core components (like delivery vectors and base editors) and customize others — enabling personalization without the need to reinvent foundational technologies for each patient.
On a different but equally transformative front, Srivastava’s team at Christian Medical College and Center for Stem Cell Research, Vellore, India, in collaboration with Emory University, developed and clinically tested a novel lentiviral gene therapy using autologous CD34+ hematopoietic stem cells to treat severe hemophilia A. The Indian government and an academic center supported the trial, which not only delivered clinically meaningful levels of endogenous factor VIII but also highlighted innovative design elements, such as a macrophage-directed promoter and an optimized factor VIII transgene (ET3).
Taken together, these studies signal a democratization of gene therapy, as researchers adapt complex technologies for personalized use and deploy them globally through cooperative frameworks. Whether through agile regulatory mechanisms that enable compassionate-use cases in the US, or through infrastructure supported by non-profit missionary hospitals and the Indian government, the scientific community is actively sharing these advances—enabled by global scientific exchange, training programs, and shared technical platforms.
In essence, the convergence of precise molecular tools, modular therapy design, and inclusive international collaboration is redefining what is possible in medicine.
As I read these papers on gene therapy, I can’t help but reflect on a similar evolution in bone marrow transplantation. In the early days, the field resembled the Wright brothers’ first flight—groundbreaking, experimental, and fraught with risk. Clinicians had few donor options to work with, outcomes varied widely, and complications arose frequently.
Today, HCT resembles modern commercial aviation: safer, faster, and widely accessible. Global donor registries like NMDP, better HLA typing, RIC, supportive care, and data sharing and reporting by CIBMTR have dramatically expanded access and improved survival, making transplants a routine, life-saving therapy rather than a last-resort experiment.
Bone marrow transplant physicians are uniquely poised to lead in the research and delivery of innovative cell and gene therapies, much like veteran explorers charting new frontiers using maps they helped create. Their deep expertise in immunology, GVHD, conditioning regimens, and cellular manipulation positions them at the intersection of science and clinical care. Just as they once advanced transplant outcomes from high-risk to standard of care, they now bring the infrastructure, clinical insight, and translational agility needed to shepherd next-generation therapies—from CAR-T to in vivo gene editing—into everyday clinical reality.
References
1.Musunuru K, Grandinette SA, Wang X, et al. Patient-specific in vivo gene editing to treat a rare genetic disease. New England Journal of Medicine. 2025;392(22):2235-2243. doi:10.1056/NEJMoa2504747
2.Srivastava A, Abraham A, Aboobacker F, et al. Lentiviral gene therapy with CD34+ hematopoietic cells for hemophilia A. New England Journal of Medicine. 2025;392(5):450-457. doi:10.1056/NEJMoa2410597
CIBMTR Welcomes Wasay Khan, MD
CIBMTR and the MCW Data Science Institute (DSI) are delighted to welcome Wasay Khan, MD, as an Assistant Professor.
Dr. Khan will serve as Scientific Director for Clinical Informatics for CIBMTR, where he will provide scientific leadership and clinical informatics support to the transformation of CIBMTR’s data and systems.
He will also serve as the Associate Director of Clinical Informatics for DSI. In that role, he will develop a plan for clinical informatics research and serve as a key liaison to MCW’s healthcare partners.
Dr. Khan is an experienced clinical physician and informatics professional with a focus on clinical medicine, biomedical informatics, genetics, and clinical trials. He earned his medical degree from Dow University of Health Sciences and completed a master’s in biomedical informatics at the University of Chicago.
As a postdoctoral fellow in the Department of Genetic Medicine at Vanderbilt University Medical Center, his research focused on clonal hematopoiesis mutations, integrating hematology, genetics, statistics, and computational biology.
Dr. Khan has also worked at the intersection of clinical informatics and industry, leveraging real-world data from large-scale datasets to accelerate drug development and inform clinical decision making at Norstella. His work supports efficient, patient-centered solutions across the healthcare ecosystem.
Najla El Jurdi, MD, to Serve as Scientific Director of Graft-versus-Host Disease Working Committee
CIBMTR proudly announces the appointment of Najla El Jurdi, MD, as the new Scientific Director of the Graft-versus-Host Disease Working Committee, effective July 1, 2025. The committee also bids farewell to Stephanie Lee, MD, MPH, who has served in this role since 2021. Dr. Lee will continue as Co-Scientific Director of the Immunobiology Working Committee.
Dr. El Jurdi is an Associate Research Physician and Head of the GVHD and Late Effects Unit at the National Cancer Institute. She received her MD from the American University of Beirut and was previously a faculty member at the University of Minnesota from 2018-2024.
Dr. El Jurdi has extensive experience with clinical trials in acute and chronic GVHD, translational research in the role of the microbiome, and retrospective analysis of GVHD data. For the past two years, she has been a CIBMTR Page Scholar working with the GVHD Working Committee. Dr. El Jurdi looks forward to working with the GVHD Working Committee Co-Chairs, statisticians, study principal investigators, and the wider transplant community to advance knowledge about GVHD. When she is not working, she enjoys exploring the parks and beaches of the greater Washington, D.C.; Maryland; and Virginia area with her two young children.
“It was a great privilege to be a CIBMTR Page Scholar and to be mentored by Stephanie (Lee) and Steve (Spellman). I’m honored to continue contributing to the transplant community in this role and look forward to being part of the CIBMTR team,’ says Dr. El Jurdi.
Please help us welcome Dr. El Jurdi!
CIBMTR Working Committees: Call for Proposals
CIBMTR and ASTCT will host the 2026 Tandem Meetings I Transplantation and Cellular Therapy Meetings of ASTCT and CIBMTR February 4-7, 2026, in Salt Lake City, Utah. During the annual meetings, CIBMTR determines its working committee study portfolio for the coming year. We invite you to submit study proposals for consideration prior to the meetings.
Proposals must be submitted to CIBMTR via the proposal form by 11:59pm PST on September 22 to be considered for discussion at a working committee meeting. Please note that the submission deadline is earlier than in previous years – September 22 – due to the earlier timing of the 2026 Tandem Meetings. CIBMTR will not accept proposals after the deadline. Please refer to the Study Proposal guidance when preparing your proposal. The working committee you select in your proposal form will review your proposal. Please refer to the working committee descriptions (click on each working committee to read the description) when considering your selection. Please contact the leadership of the specific working committee to which you wish to submit if you have additional questions.
CIBMTR reviews new proposals based on scientific merit and ability to move the field forward, study question timeliness, feasibility, and our ability to complete the study in a timely fashion. Working committee and CIBMTR leadership, as well as committee members (through proposal scoring), play a role in determining which studies are chosen.
Please contact us at CIBMTRStatsOps@mcw.edu with any questions or if you wish to become a working committee member.
CIBMTR Reaches PRO Protocol Milestone
CIBMTR is excited to announce that we reached a major milestone on the CIBMTR PRO Protocol!
 More than 5,000 patient-reported outcomes (PRO) surveys have now been collected from 1,700+ patients across 43 centers.
More than 5,000 patient-reported outcomes (PRO) surveys have now been collected from 1,700+ patients across 43 centers.
This major achievement highlights our continued dedication to advancing cell therapy and our active effort to improve patient outcomes. These valuable data insights are now available through the CIBMTR Research Database Protocol.
Explore the data:
Visit the PRO Protocol webpage for an updated data listing. The data are available by patient demographics and PRO time points.
Interested in using these data?
We invite you to join our collaborative research community by proposing a CIBMTR Working Committee Study.
Your involvement will help advance the PRO protocol’s mission to capture patient-reported symptoms and functioning —driving forward patient-centered research in cell and gene therapy.
Questions?
If you’d like to learn more about PRO data, or to ensure your patients are included in the PRO Protocol, please reach out to our study team at Surveys@cibmtr.org.
Welcome, 2025 Page Scholar Participants!
We are excited to introduce our second class of the CIBMTR Page Scholar Program! CIBMTR developed the Page Scholars Program in 2023 as the Working Committee Training and Leadership Program (WCTL) with the aim of integrating early career investigators into the workings of CIBMTR and the working committees. CIBMTR changed the program name to remember Kristin Page, one of the founders of the program, in honor of her dedication to teaching and promoting career development, research, and leadership skills, which are hallmarks of the program. We hope to build on the success of the inaugural class as we welcome the 2025 Page Scholars!
We received an overwhelming response to the program, receiving applications from 59 early career investigators. The candidates were all reviewed by Page Scholars Leadership, CIBMTR Scientific Directors, and Nominating Committee, and they were approved by the Advisory Committee. The 9 participants selected all share a high level of dedication, achievement, and enthusiasm for clinical research. We warmly welcome them to the program and are excited to be a part of their career development. We will be highlighting the participants and the Page Scholars Program in upcoming editions of this newsletter.
 |
 |
 |
| Xia Bi, MD, MS Thomas Jefferson University Leukemia Working Committee |
Ryan Stubbins, MD, MS University of British Columbia Leukemia Working Committee |
Arpita Ghandi, MD, MS Oregon Health and Sciences University GVHD Working Committee |
 |
 |
 |
| Sanghee Hong, MD Duke University Donor and Recipient Health Services Working Committee |
Taymour Hammoudi, MD, PhD University of Colorado Immunobiology Working Committee |
Poorva Bindal, MD UMass Chan Medical School Morbidity, Recovery and Survivorship Working Committee |
 |
 |
 |
| Reena Jayani-Kosarzycki, MD Vanderbilt University Morbidity, Recovery and Survivorship Working Committee |
Jane Koo, MD Cincinnati Children’s Hospital Non-Malignant Diseases Working Committee |
Takuto Takahashi, MD, PhD Boston Children’s Hospital / Dana-Farber Cancer Institute Pediatric Cancer Working Committee |
Congratulations to Our Inaugural Page Scholar Participants!
It’s graduation day!
For many young adults, it’s the time of year for graduation – from high school, college, residency, and fellowships. And for our Page Scholars, the graduation of our inaugural class of this program. Each of the 8 participants has spent the past 2 years diving in to learn about CIBMTR and helping to lead the working committees. We give an immense THANK YOU to each of the Page Scholars for all their hard work, dedication, and passion for CIBMTR and the research potential that it holds. We truly enjoyed learning with you and can’t wait to see what your careers hold!
| Page Scholar | Working Committee and Achievements |
|---|---|
|
Brian Ball, MD |
Non-Malignant Diseases Working Committee
|
|
Zeinab El Boghdadly, MD |
Infection and Immune Reconstitution Working Committee
|
|
Najla El Jurdi, MD |
GVHD Working Committee
|
|
Hany Elmariah, MD, MS |
Chronic Leukemia Working Committee
|
|
Megan Herr, PhD |
Donor and Recipient Health Services Working Committee
|
|
Mariam Nawas, MD |
Acute Leukemia Working Committee
|
|
Jennifer Saultz, DO |
Immunobiology Working Committee
|
|
Michelle Schoettler, MD |
Morbidity, Recovery and Survivorship Working Commmittee
|
Immunobiology Working Committee

Pictured left to right: Esteban Arrieta-Bolaños, Cara Benjamin, Meilun He, Yung-Tsi Bolon, Jennifer Saultz, Brian Betts, and Tao Wang.
Committee Leadership
Co-Chairs:
- Esteban Arrieta-Bolaños, University Hospital Essen, Germany
- Brian Betts, Roswell Park Comprehensive Cancer Center, Buffalo, NY
- Cara Benjamin, University of Miami/Sylvester Comprehensive Cancer Center, Miami, FL
Scientific Director:
- Stephanie Lee, Fred Hutchinson Cancer Center, Seattle, WA
- Yung-Tsi Bolon, CIBMTR NMDP, Minneapolis, MN
Statistical Director:
- Tao Wang, CIBMTR MCW, Milwaukee, WI
Statistician:
- Meilun He, CIBMTR NMDP, Minneapolis, MN
CIBMTR Page Scholar (not pictured):
- Taymour Hammoudi, Children’s Hospital Colorado, Aurora, CO
With more than 180 scientific papers, the Immunobiology Working Committee is CIBMTR’s largest committee by study volume. Since our last newsletter article in 2024, we said goodbye and thank you to Shahinaz Gadalla (Working Committee Co-Chair) and Jennifer Saultz (Page Scholar Program participant), and we now welcome Taymour Hammoudi (Page Scholar Program participant) to the committee.
The Immunobiology Working Committee conducts studies aimed at understanding the complex genetic, epigenetic, and immune molecular and cellular interactions involved in transplantation—and how these factors influence patient outcomes post-transplant, including major and minor histocompatibility complex matching, natural killer and T cell repertoire, cytokines / chemokines, genomics, and immune-response determinants. The committee also compares clinical outcomes across different donor types to inform donor choice (e.g., mismatched related versus unrelated donors) and explores novel biostatistical and analytic approaches to investigate the impact of various types of HLA mismatches. Given the experimental and functional nature of this research, approximately one-third of the committee’s studies require sample testing. View the current sample inventory and request samples from the Immunobiology Research Program by following the instructions available on cibmtr.org.
The Immunobiology Working Committee currently has 11 studies in progress, including collaborations with research partners such as the National Cancer Institute. Current investigations include HLA evolutionary divergence in matched unrelated donor transplants, 6-locus HLA immunopeptidome divergence in mismatched unrelated donor transplants, the effects of HLA-DP mismatching in the era of post-transplant cyclophosphamide, and donor selection in haploidentical transplantation for aplastic anemia. Several high-impact studies published in the past two years have also delivered valuable insights, including:
- Studies have shown the importance of selecting younger donors, in a range of situations and different types of donors, to improve outcomes:
- Patients with a score of 0 using a novel peptide binding score based on physicochemical properties of mismatched amino acids in the HLA antigen-recognition domain had less transplant-related mortality than pairs with higher scores:
- About 3% of patients with aplastic anemia have pathogenic or likely pathogenic mutations in HLH genes, but these mutations are not associated with adverse outcomes after transplantation:
The Immunobiology Working Committee continues to demonstrate a strong publication record, with its investigators publishing 10 manuscripts since May 2024 in journals such as Transplantation and Cellular Therapy, British Journal of Haematology, and Blood Advances. A complete list of the committee’s publications is available online.
Successful proposals start with our research community members. We depend on bold clinical and scientific questions backed by strong preliminary data and testable hypotheses. Your diverse perspectives and expertise—from basic immunobiology to translational and clinical science—are what fuel our progress. Therefore, we encourage investigators to submit innovative proposals during the solicitation period (deadline: September 22, 2025). We also gather annually at the Tandem Meetings of ASTCT and CIBMTR, with additional opportunities to connect throughout the year. We encourage all investigators with an interest in immunology, immunobiology, or human genetics to get involved with this committee. Feel free to contact leadership or staff to learn more or to discuss your research ideas and proposals. We look forward to collaborating with you and seeing you at upcoming meetings!
Pediatric Cancer Working Committee
 |
 |
 |
| Akshay Sharma | Parinda Mehta | Christine Phillips |
 |
 |
|
| Larisa Broglie | Kwang Woo Ahn | |
 |
 |
|
| Sarthak Kumar | Takuto Takahashi |
Committee Leadership
Co-Chairs:
- Akshay Sharma, St. Jude Children's Research Hospital, Memphis, TN
- Parinda Mehta, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
- Christine Phillips, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Scientific Director:
- Larisa Broglie, CIBMTR MCW, Milwaukee, WI
Statistical Director:
- Kwang Woo Ahn, CIBMTR MCW, Milwaukee, WI
Statistician:
- Sarthak Kumar, CIBMTR MCW, Milwaukee, WI
CIBMTR Page Scholar:
- Takuto Takahashi, MD, PhD, Dan Farber and Boston Children’s Hospital, Boston, MA
What does the Pediatric Cancer Working Committee do?
The Pediatric Cancer Working Committee provides scientific oversight for studies related to allogeneic and autologous HCT for children, adolescents, and young adults with cancer. Because transplantation approaches differ from those used in adults, researchers must independently evaluate pediatric patients to improve transplant outcomes for this population.
What studies are currently in progress with the Pediatric Cancer Working Committee?
The Pediatric Cancer Working Committee portfolio includes 9 studies in various stages of completion.
Protocol Development: After the working committee accepts a study, the protocol is discussed and modified collaboratively by PIs, statisticians, statistical directors, and working committee leadership. Statisticians then clean the CIBMTR data and build demographic tables to support final protocol decisions. After the protocol is reviewed at a CIBMTR Statistical Meeting and receives feedback from the working committee via email, then it is considered final.
- PC19-02 Does mixed peripheral blood T cell chimerism predict relapse? (A Lake / S Prockop / J Boelens / K Peggs)
- CT20-02 Resource utilization with CAR T-cells (M Battiwalla/ H Rangarajan/ C Scheckel)
- PC22-02 Evaluating predictors of access and outcomes with HCT in pediatric and adolescent patients with relapsed / refractory classical Hodgkin lymphoma after treatment on an initial cooperative group clinical trial (S Castellino/ J Kahn)
- PC23-01 Post-transplant cyclophosphamide vs. TCR αβ/CD19+ deplete approaches for haploidentical transplant in pediatric patients with acute leukemias and MDS: A CIBMTR/EBMT collaborative study (A Li/ H Rangarajan/ P Satwani)
- PC24-01 Transplantation and cellular therapy for children and young adults with down’s syndrome and acute leukemia (L Appell/ S Rotz)
Analysis: Once the protocol is finalized, statisticians clean the outcomes data, and statistical directors complete the analysis. The team then reviews the results at a CIBMTR Statistical Meeting and circulates them to working committee members who provided feedback on the protocol.
- PC20-02 Germline genetics of pediatric MDSJ (Poynter/ L Spector).
- PC23-02 Comparison of bone marrow and PBSC as graft source in children undergoing allogeneic HCT for hematological malignancies with unmanipulated haploidentical grafts utilizing post-transplant cyclophosphamide as GVHD prophylaxis (A Srinivasan/ J Krueger).
Presentation and Manuscript Preparation: Researchers presented the following studies at national or international meetings, and they are currently preparing manuscripts for publication. They will circulate the manuscripts for feedback to individuals who submitted comments during the protocol and analysis stages.
- PC19-03 The impact of pre-transplant extramedullary disease on the outcome of allogeneic HCT for AML in children (H Rangarajan/ P Satwani /D Chellapandian). Poster presentation at ASH in December 2024
- PC22-01 Impact of GVHD following allogeneic HCT on leukemia free survival in hematologic malignancies within the pediatric disease risk index risk stratification (A Bauchat/ M Qayed). Oral Presentation Tandem 2025
Were any new studies selected at the 2025 Tandem Meetings?
Investigators submitted 12 proposals to the Pediatric Cancer Working Committee, and 7 were presented at the Tandem Meetings in February 2025. Based on feasibility and feedback from working committee members at the meeting, the committee selected 1 study to move forward:
- Impact of planned post-transplant granulocyte colony stimulating factor on transplant-related outcomes in pediatric patients with malignant disease undergoing HCT with post-transplant cyclophosphamide for GVHD prophylaxis
What other activities is the Pediatric Cancer Working Committee involved in?
The committee seeks to be a resource for pediatric transplant and cellular therapy providers. To enhance our ability to conduct practice-changing research, CIBMTR’s Pediatric Cancer Working Committee is working on strengthening our collaboration with the EBMT Pediatric Diseases Working Party. We are working together to establish a streamlined approach for studies combining both CIBMTR and EBMT databases. PC24-01 (Transplantation and cellular therapy for patients with Down syndrome) is a testament to this collaborative effort.
CIBMTR and the Children’s Oncology Group (COG) are actively collaborating to merge resources through PC22-02 (Evaluating predictors of access and outcomes with HCT in pediatric and adolescent patients with relapsed / refractory classical Hodgkin lymphoma). CIBMTR is also working with investigators in the Pediatric Acute Leukemia (PeDAL) program to integrate transplant outcomes into that initiative.
Both partnerships will hopefully set the stage for future collaborative efforts as well.
How can I get involved with the Pediatric Cancer Working Committee?
The success of our committee depends on new ideas, testable hypotheses, and participation by individuals with different perspectives and scientific backgrounds. Having an engaged group of investigators allows us to conduct impactful studies. We encourage colleagues with an interest in research with CIBMTR and the Pediatric Cancer Working Committee to reach out to this committee’s leadership to discuss ideas and collaboration. If you are interested in joining the committee, contact the Statistical Operations group at CIBMTRStatsOps@mcw.edu.
The Pediatric Cancer Working Committee encourages all investigators with an interest in pediatric cancer to propose studies through CIBMTR’s Propose a Working Committee Study webpage. Independent researchers can also download and analyze datasets from prior studies, which are available on the Publicly Available Datasets webpage on CIBMTR’s website.
More information about this committee is available online.
Save the Date for the 2026 Tandem Meetings!
By Alicia Halfmann and Maira Brey
The 2026 Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR will be held February 4-7, 2026, with pre-conference events on Tuesday, February 3, in Salt Lake City, Utah.
Current details about the 2026 Tandem Meetings are available on our website at TandemMeetings.com, including:
- Educational content through the online agenda
- Abstract submission information
- Exhibit and support opportunities
- Registration and housing (expected to open in fall 2025)
If you have any questions, feel free to contact us at TandemMeetings@mcw.edu.
Stay connected by following ASTCT and CIBMTR on social media as well as the official hashtag, #Tandem26, for the latest updates.
Spotlight on CIBMTR at ISOQOL 2025: Advancing Patient-Centered Research in Cellular Therapy
A symposium at the 2025 International Society for Quality of Life Research (ISOQOL) Conference in Milwaukee, WI, will feature CIBMTR, highlighting the integration of PROs into clinical registries for HCT and cellular therapy, including HCT and CAR-T.
The symposium, titled “PRO be nimble, PRO be quick,” will explore the development and impact of CIBMTR’s centralized PRO infrastructure—an initiative that has enrolled more than 1,800 patients across 42 centers since its launch in 2020. This effort aims to enhance understanding of patient experiences, symptoms, and quality of life throughout the transplant and survivorship journey.
Rachel Cusatis, PhD, will moderate this session, which includes three presentations:
- Deborah Mattila, Survey Research Group Manager, will discuss the design and implementation of the PRO system, including strategies to address disparities in response rates and improve outreach to underrepresented populations.
- Miranda Kapfhammer, PRO Clinical Research Coordinator II, will present findings on financial toxicity among patients prior to receiving HCT or CAR-T therapy, revealing significant disparities by age, race/ethnicity, and income, and their associations with mental health.
- Rachel Cusatis, PhD, Senior Scientific Director of Patient-Centered Research and Survivorship, will compare real-world PRO data with clinical trial participants from the ACCESS study, highlighting differences in social vulnerability and access to care among patients receiving similar treatments.
This symposium highlights the power of real-world PRO data to uncover disparities, guide interventions, and advance patient-centered care in cellular therapy. It also aligns with the broader conference theme, “Artificial Intelligence & the Future of Quality of Life Research,” by demonstrating how digital platforms and centralized infrastructure can amplify the patient voice in clinical research. CIBMTR’s own Kathryn Flynn, PhD, is a Scientific Program Committee Co-Chair. The 32nd Annual ISOQOL Conference will take place at the Hyatt Regency in Milwaukee, WI, October 22-25, 2025.
Additionally, CIBMTR will present a number of abstracts at the conference:
| Presentation Title | Date / Time | Presentation Type | Presenter |
|---|---|---|---|
| Using self-reported measures to assess financial toxicity in cellular therapy patients with hematologic diseases in a US setting: A scoping review | Oct. 23, 2025
9:45 a.m. |
Poster | Sarah Reed-Thryselius, MPH |
| Financial toxicity and health: Results from a U.S. nationally representative survey | Oct. 23, 2025
2 p.m. |
Oral | Oyiza Usman |
| Evaluation of demographic DIF in the PROMIS and EORTC cognitive function measures in a nationally representative US sample | Oct. 24, 2025
1:35 p.m. |
Oral | Miranda Kapfhammer |
| Evaluation of enrollment rates to PRO data collection in a large clinical registry in the US - the first 5 years | Oct. 24, 2025
3:35 p.m. |
Oral | Idayat Akinola |
| Individual and community-level social determinants associated with representation in a cellular therapy registry | Oct. 24, 2025
3:35 p.m. |
Oral | Carlos Litovich, MPH |
Implementation Science
Seventeen years—that’s the average time it takes for evidence to change practice. CIBMTR’s Implementation Science team is committed to changing that timeline. By translating research findings into real-world policy and care faster, they are working to improve outcomes and ensure patients benefit from scientific progress in a more timely manner.
CIBMTR’s Implementation Science focus areas and example projects include:
- Understanding Current Practice: Conducting studies and analyses to understand the current state of evidence-based practices and potential barriers to adoption.
- With increasing options for source selection for allogeneic transplant, researchers conducted a qualitative study with 50 transplant centers to identify factors influencing transplant centers’ donor and cord blood unit selections.
- Disseminating Research: Sharing research findings with specific audiences to improve awareness of the results.
- The team promotes results beyond publications through summaries for both clinicians and patients, including clinical summaries and patient summaries.
- Supporting Implementation: Designing and deploying implementation strategies to enhance the uptake of evidence-based practices.
- Five centers are currently testing a toolkit (as part of the ASTCT-NMDP ACCESS Initiative) for enhancing access to care through quality improvement projects.
What is a CRO and What Does It Do?
A contract research organization (CRO) assists sponsors with conducting a clinical study. This can include supporting the entire clinical study starting from protocol design all the way through publishing the data, or the CRO could support only certain aspects of the study for which the sponsor needs some extra help or specific expertise.
CIBMTR’s CRO Services team operates as a niche CRO with expertise in supporting clinical studies for transplantation, cellular, and gene therapies. We support both industry-sponsored studies and multi-center investigator-initiated studies, which industry partners, federal grants, or charitable foundations may fund. This funding includes both comprehensive support packages and à la carte services, covering everything from study design and biospecimen tracking to final trial results reporting. For more than two decades, our team has gained expertise in overseeing complex multicenter trials taking place in our substantial network of 250 transplant sites. CIBMTR CRO Services currently manages 26 active clinical studies in whole or in part, with more than 45,000 people participating in our studies since 2001.
CIBMTR’s CRO Services team includes several specialized functional groups, each bringing expertise that contributes to the success of a clinical study. Below is an overview of these functional groups and their roles in supporting clinical research:
1. Clinical Project Management
Clinical project managers lead cross-functional study support teams that include clinical research associates (study monitors), start-up specialists, and clinical trial assistants as well as experts from any other relevant operational areas. The teams’ work ensures all study activities are compliant with FDA regulations and good clinical practice (GCP).
Responsibilities of the study support teams include:
- Training sites on protocol requirements
- Facilitating system access
- Collecting study documentation
- Upholding human subject protection policies
- Overseeing safety monitoring processes
- Maintaining continuous audit readiness
This team also works closely with scientific experts to design trials that mirror real-world clinical practice, giving each trial the best chance of success. Clinical project managers continuously evaluate efforts to meet study milestones and sponsor expectations per the study protocol and contract.
2. Data Management, Safety, and Statistical Analysis
The data management team creates data collection forms within an electronic data capture (EDC) system, such as Veeva, ensuring that sites can provide the required study data. The team also configures the database with study-specific automated data validation checks and supports sites in managing data entry requests.
Study statisticians collaborate closely with the data management and broader study teams to assess protocol feasibility and determine an appropriate sample size. They then develop a study-specific statistical analysis plan and conduct analyses at pre-specified time points throughout the study.
The safety specialist oversees the processing and documentation of safety events. The safety team works closely with the study team to monitor adverse events reported by sites in real time via the EDC system. This team carefully reviews and, when necessary, codes all safety events to ensure alignment with standardized reporting terminology, event naming, and grading criteria.
These data management, safety, and statistical analysis teams within a study team work collaboratively to format study data according to submission requirements for the Data and Safety Monitoring Board, IRB, FDA, and final study publications.
3. Sample Management and Survey Research Group
Some studies require the collection of additional participant data, such as biological samples or PRO surveys. The sample management team oversees all aspects of sample-related logistics, beginning with protocol development input and study-specific sample collection procedures. Their responsibilities span the entire study and include managing collection timing, shipping logistics, destination tracking, receipt confirmation, and processing methods to prevent sample loss or mishandling. The team also manages the ordering and distribution of collection and shipping kits and maintains oversight of central lab and pharmacy coordination to ensure consistency in sample handling across all study sites.
The Survey Research Group collects and manages PRO data, storing it in a separate system. Rather than relying on clinical staff reports, the team gathers this information directly from participants—via phone outreach or through self-administered surveys. These firsthand responses offer a valuable perspective on participants’ quality of life and the mental and emotional impact of treatment.
4. Quality Assurance and Compliance
To ensure high data quality, clinical research associates (CRAs) or monitors review data entered by sites into EDC data collection forms and verify those data against source information in the medical records. CRAs may conduct these reviews either onsite (at hospitals or clinics) or remotely. Leveraging their clinical expertise in transplantation, study CRAs are well-equipped to assess site compliance with the study protocol and regulatory requirements, particularly within the complex landscape of HCT and cellular therapy.
Study staff must periodically collect and verify additional regulatory documentation. To support this process, monitors collaborate closely with the clinical project manager and clinical quality specialist to ensure accurate record-keeping and continued compliance with study procedures and regulatory standards.
What sets CIBMTR CRO Services apart from other CROs:
- Specialized focus on HCT and cell and gene therapy clinical studies
- Scientific advisors with decades of experience and access to real-world transplant data, providing critical insights to inform robust clinical trial design
- Established contracts and strong relationships with more than 250 clinical sites
- Comprehensive infrastructure to support IRB submissions, Data and Safety Monitoring Board oversight, and EDC platform integration
The Registry of Unmet Need (RUN): An Analysis of Patients Without an HLA-Matched Donor in the Global Database
By Valerie Stewart, MS, PhD
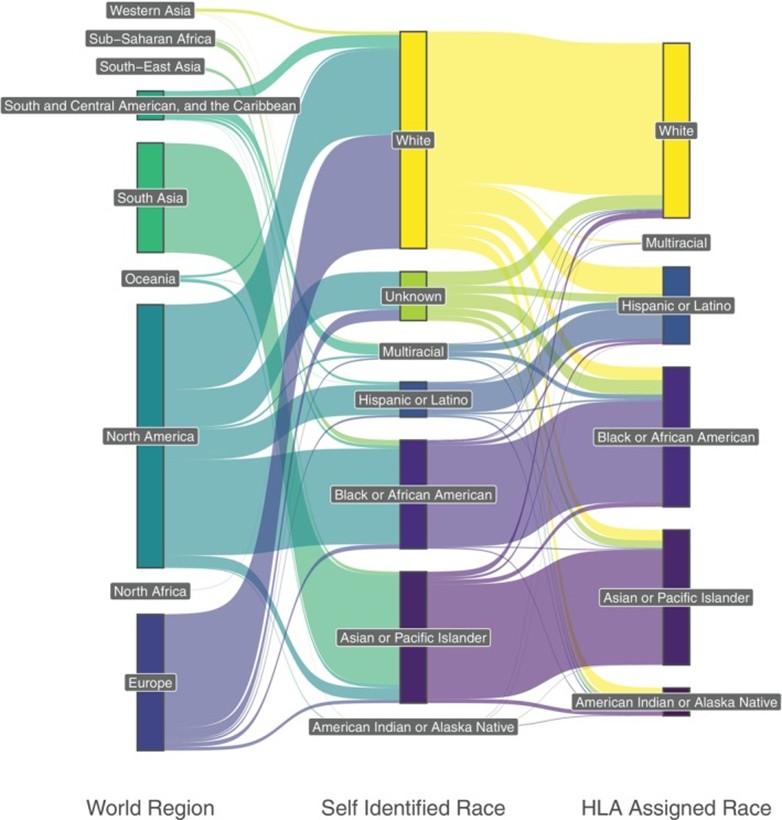
A three‐level Sankey diagram of (left) the regional origin of the RUN patients, (middle) the self‐assigned or presumed race category and (right) the population category assigned using an HLA‐based classifier (15 patients assigned to multiple population categories are not displayed).
The WMDA is a global organization that manages a database of unrelated donors and cord blood units from international registries and biobanks that may be queried on behalf of patients seeking HCT. Despite an inventory of more than 43 million cell sources, transplant teams are still unable to identify HLA-matched adult donors (MUDs) for some patients. For this reason, WMDA launched the Registry of Unmet Need (RUN), a project designed to identify global deficits in HLA coverage to better serve the international community. Researchers recently published the results of this initiative (Maiers et al, 2025).
In 2017, WMDA invited its members to share HLA profiles of patients for whom no MUD, defined as an 8/8 match at HLA-ABCDRB1, existed at the time of search onset (2015-2017). Sixteen registries in 15 countries submitted HLA profiles of 3,646 patients with reported geographic ancestries spanning 53 countries. Researchers evaluated these genotypes using bioinformatics approaches to identify gaps in global donor inventory, which at the time included more than 33.5 million donors.
Researchers analyzed patient HLA genotypes to determine why a MUD could not be identified. They ruled out rare HLA alleles as the cause since the cohort included only twelve across all loci. When evaluating race and ethnicity, researchers observed that patients’ reported race and imputed HLA-based race did not consistently align with the geographic regions from which they initiated their donor searches (see Figure). Patients from North America demonstrated greatest diversity in assigned race and showed a smaller proportion of White patients having reported race alignment with HLA assignment, suggesting greater genetic diversity and more complex genetic admixing than anticipated. The main barrier to identification of a MUD was having haplotypes with low-frequency relative to a control population for whom multiple MUD existed; most RUN patients had two low-frequency haplotypes.
Researchers also examined the likelihood that a newly-added donor would match the patient by repeating patient searches in 2024; while the global inventory increased by almost 10 million donors, only 11% of RUN patients now have one or more likely MUD. RUN patients with new matches had a median genotype frequency 11.6 times higher than those who did not, with matches more likely to come from diverse populations in historically underserved regions of the world.
While these findings support the ongoing, strategic recruitment of genetically diverse donors , they underscore that increased recruitment alone cannot fully meet the needs of the entire global patient population. When transplants are delayed, complications may arise— and if a matched donor hasn’t already been identified, even exhaustive searches or targeted outreach are unlikely to succeed. A prolonged search for a MUD is not advisable considering the availability of alternative graft sources, including cord blood, haploidentical, and mismatched donors. With the success of MMUD in HCT demonstrated by CIBMTR trials, including 15-MMUD and ACCESS, MMUD can offer both expanded access to transplant and facilitate donor selection based on favorable criteria, including age. The WMDA remains committed to providing unrelated donor options. Leveraging findings from the RUN study, it will continue to support the strategic global recruitment of donors to enrich the global database. These efforts aim to ensure that all patients have access to cell therapy. In addition, the WMDA will empower transplant search coordinators with the knowledge to select the best cell source for their patients.
Reference
Maiers M, Greco-Stewart V, Madbouly A, Robinson J, Eberhard HP, Sauter J, Louzoun Y, Israeli S, Bolon YT, Pingel J, Schmidt AH, Spierings E, Leonhard-Melief C, Foeken L, Schuit M, Venter A, Marsh SGE. The Registry of Unmet Need: A World Marrow Donor Association Analysis of Patients Without an HLA Match. HLA. 2025 May;105(5):e70255. doi: 10.1111/tan.70255. PMID: 40404178; PMCID: PMC12097852.)
BMT CTN Active Trials and Recent Publications
By Mykala Heuer, BSN, RN
The BMT CTN is in its fifth grant cycle and has now enrolled more than 17,250 patients. Established in 2001, the Network is funded by the NHLBI and NCI. 
Clinical Trials: Open Enrollment
The BMT CTN encourages widespread transplant community participation in clinical trials. If your center is interested in participating, please visit the BMT CTN website.
There are 8 active BMT CTN trials. Of the BMT CTN-led trials, there are 2 protocols open to accrual and 4 are in development. The following BMT CTN protocols are open to accrual:
- BMT CTN 2203 – A randomized, multicenter, Phase III trial of tacrolimus / methotrexate / ruxolitinib versus post-transplant cyclophosphamide / tacrolimus / mycophenolate mofetil in non-myeloablative / reduced intensity conditioning allogeneic peripheral blood stem cell transplantation
- BMT CTN 2207 – A Phase II trial of non-myeloablative conditioning and transplantation of haploidentical related, partially HLA-mismatched, or matched unrelated bone marrow for newly diagnosed patients with severe aplastic anemia
- This trial also offers a proactive financial navigation service as part of a BMT CTN Access to Clinical Trials initiative.
If your center is participating in BMT CTN 2203 or BMT CTN 2207, please consider participating in BMT CTN 2302 - Facilitating Activation of Study Trials (FAST), which is a time-and-motion study to understand the infrastructure, processes, barriers, and effective and ineffective center practices related to activation of a cooperative group trial.
BMT CTN Publications
There are 197 published BMT CTN articles, including 47 primary analyses. Researchers published 2 primary manuscripts in 2025:
- 1507 Adult Cohort: Kassim et al. Haploidentical bone marrow transplantation for sickle cell disease. NEJM Evidence. 2025 Mar 1; 4(3):EVIDoa2400192. doi: 10.1056/EVIDoa2400192. Epub 2025 Feb 25.
https://pubmed.ncbi.nlm.nih.gov/39998298/ - 1704: Sorror et al. Novel composite health assessment risk model for older allogeneic transplant recipients: BMT-CTN 1704. Blood Advances. 2025 Jul 8; 9(13):3268-3280. doi: 10.1182/bloodadvances.2025015793. PMID: 40101246.
https://pubmed.ncbi.nlm.nih.gov/40101246/
About the BMT CTN
CIBMTR shares administration of the BMT CTN Data and Coordinating Center with NMDP and The Emmes Company. Together, these three organizations support all BMT CTN activities. The BMT CTN Steering Committee is currently under the leadership of John Levine, MD, (Mount Sinai) as Steering Committee Chair; Stephanie Lee, MD, (Fred Hutchinson Cancer Center) as Steering Committee Chair-Elect; and Miguel-Angel Perales, MD, (Memorial Sloan Kettering Cancer Center) as Steering Committee Vice-Chair.
To get up-to-date information about BMT CTN studies, meetings, and news, be sure to follow us on X (Previously known as Twitter): @BMTCTN
Publicly Available Datasets
 View a summary of the Publicly Available Datasets and the data dictionary containing the most commonly used variables.
View a summary of the Publicly Available Datasets and the data dictionary containing the most commonly used variables.
In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, CIBMTR makes the final datasets from published studies publicly available on CIBMTR’s Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
While providing these data, CIBMTR is committed to safeguarding the privacy of participants and protecting confidential and proprietary data. Upon accessing the datasets page on CIBMTR’s public website, the viewer is notified that the dataset was collected by CIBMTR, and CIBMTR’s supporters are listed. The webpage also clearly notes the terms and conditions of dataset usage.
NEW datasets are now available online.
Share Research in Plain Language
By Jennifer Motl
These new plain-language summaries of CIBMTR research may help your patients:
|
|
Transplant helps teens, young adults with sickle cell disease; read more: |
|
|
Half-matched transplant helps adults with sickle cell disease; read more:
|
 |
Partially matched blood stem cell transplant is safe and effective; read more: |
Find more summaries at cibmtr.org/summaries.
Our Supporters
CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; 75R60222C00008, 75R60222C00009, and 75R60222C00011 from the Health Resources and Services Administration (HRSA); and N00014-24-1-2057 and N00014-25-1-2146 from the Office of Naval Research.
Additional federal support is provided by OT3HL147741, P01CA111412, R01CA100019, R01CA218285, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01FD008187, R01HL171117, R21AG077024, U01AI069197, U01AI184132, U24HL157560, and UG1HL174426.
Support is also provided by Boston Children’s Hospital; Fred Hutchinson Cancer Center; Gateway for Cancer Research, Inc.; Jeff Gordon Children’s Foundation; Medical College of Wisconsin; NMDP; Patient Center Outcomes Research Institute; PBMTF; St. Baldricks’s Foundation; Stanford University; Stichting European Myeloma Network (EMN); and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune LLC; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc.; Amgen, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; Autolus Limited; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, inc.; Blueprint Medicines; Bristol Myers Squibb Co.; CareDx Inc.; Caribou Biosciences, Inc.; CSL Behring; CytoSen Therapeutics, Inc.; DKMS; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gift of Life Biologics; Gift of Life Marrow Registry; HistoGenetics; ImmunoFree; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Merck & Co.; Mesoblast, Inc.; Millennium, the Takeda Oncology Co.; Miller Pharmacal Group, Inc.; Miltenyi Biotec, Inc.; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc.; OriGen BioMedical; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc.; Sobi, Inc.; Sociedade Brasileira de Terapia Celular e Transplante de Medula Óssea (SBTMO); Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Tscan Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.