February 2026 Newsletter
Perspectives
By Sumithira Vasu, MBBS, MD
As we near the 2026 Tandem Meetings and look forward to connecting with collaborators and exploring the next frontier, I’m sharing a few ways everyone can stay engaged—whether you’re attending for the first time or you’re a seasoned participant.
With the changing regulatory landscape for cell and gene therapies, make sure to attend the ASTCT Spotlight Session: Endpoints and FDA Strategies for BMT and Cell Therapy Clinical Trials, featuring Upendra Mahat, MD, from the FDA, who will discuss endpoints in GVHD trials. Claudio Brunstein, MD, PhD, will also present during the session. If you are a clinical investigator, don’t miss this unique session designed to foster meaningful discussion. Join us for this session on Saturday, February 7, from 1:30-3 p.m. MST.
The ASTCT-CIBMTR-EBMT plenary session, “From Breakthrough to Bedside: Navigating the Surging Transplant and Cell Therapy Landscape,” highlights the challenges and opportunities of adapting to a rapidly expanding therapeutic landscape, as commercially available cell therapy expands into solid tumors and clinical trials in autoimmune diseases continue to increase. These advances create exciting options for patients, and they underscore the urgency for transplant and cell therapy clinicians and clinical investigators to strategically plan and build capacity—both in personnel and resources—to serve a growing patient population. This session will be held on Wednesday, February 4, from 8:15-10 a.m. MST.
Make sure to explore the oral and poster abstracts and attend the many meetings for ASTCT special committees. You also won’t want to miss the CIBMTR Working Committee meetings, happening every day from February 5 through February 7, for the opportunity to vote on proposals.
There are many opportunities for collaboration, and I encourage you to use this dedicated time to connect with colleagues as we enter an exciting period of unprecedented growth in the field of transplant and cell therapy.
Now Available: 2025 CIBMTR Annual Report
CIBMTR’s 2025 Annual Report is now available to view on the Administrative Reports webpage.
This document explains who we are, our strategy and impact, our research areas, how we share knowledge, and how we manage data. 2025 accomplishments are highlighted throughout the report.
Thank you for helping us make 2025 another successful year!
Pick up your copy of the 2025 Annual Report at the 2026 Tandem Meetings. (Find CIBMTR's booth within the 2026 Tandem Meetings Hub.)
Leukemia Working Committee
Committee Leadership
Co-Chairs:
- Lori Muffly, Stanford Health Care, Stanford, CA
- Mark Juckett, University of Minnesota, Minneapolis, MN
- Michael Grunwald, Levine Cancer Institute, Charlotte, NC
- Nelli Bejanyan, Moffitt Cancer Center, Tampa, FL
- Tania Jain, Johns Hopkins University School of Medicine, Baltimore, MD
- Veronika Bachanova, University of Minnesota, Minneapolis, MN
Scientific Director:
- Wael Saber, CIBMTR MCW, Milwaukee, WI
Statistical Directors:
- Kwang Woo Ahn, CIBMTR MCW, Milwaukee, WI
- Soyoung Kim, CIBMTR MCW, Milwaukee, WI
Statisticians:
- Wentong (Dylan) Liu, CIBMTR MCW, Milwaukee, WI
- Zhongyu (Nick) Feng, CIBMTC MCW, Milwaukee, WI
CIBMTR Page Scholars:
- Ryan Stubbins, BC Cancer Agency, Vancouver, BC
- Xia Bi, Thomas Jefferson University Hospital, Inc., Philadelphia, PA
The Leukemia Working Committee aims to define the optimal timing of HCT and immune effector (adoptive) cell therapy (ACT) and drives strategies to improve outcomes for patients with AML, ALL, MDS, CML, CLL, MPN, and additional rare acute and chronic leukemias. Although HCT / ACT use is expanding for many of these diseases, low prevalence limits prospective clinical trials. Of critical importance, many high-impact studies exclude patients with rare leukemias, including myelofibrosis and CML, creating gaps in evidence. To address this, the committee conducts high-quality retrospective analyses, the most robust approach to optimizing HCT / ACT strategies for chronic leukemias.
The committee leverages CIBMTR’s Outcomes Database to conduct practice-changing research in HCT / ACT for hematologic malignancies. In recent years, researchers published multiple peer-reviewed manuscripts and presented findings through both oral and poster presentations. Committee members also ensure accurate data capture by overseeing MDS and MPN forms, driving improvements in disease status and treatment response reporting.
The Leukemia Working Committee fosters new study proposals by sharing disease-specific lists of accepted studies and previously declined proposals with clear rationales. This transparency helps investigators develop feasible, nonredundant, and high-impact study proposals. Committee leadership actively engages with the transplant community to answer questions and discuss potential protocols.
Recently published studies include:
- LK21-01b Gui G, Ravindra N, Hegde PS, et al. Measurable residual mutated IDH1 before allogeneic transplant for acute myeloid leukemia. Bone Marrow Transplantation. 2025 Feb 1; 60(2):154-160. doi:10.1038/s41409-024-02447-4. Epub 2024 Nov 6. PMC11810766.
- This multicenter retrospective study evaluated whether persistence of IDH1 mutations before allogeneic HCT predicts outcomes in adults with IDH1-mutated AML in first complete remission. Using ultra-sensitive error-corrected next-generation sequencing, residual IDH1 mutations were detected in 36% of 148 patients’ pre-transplant blood samples. However, IDH1 persistence was not associated with differences in overall survival or relapse risk after transplant. In contrast, among patients with co-mutations in NPM1 and/or FLT3-ITD, persistence of those mutations, rather than IDH1 alone, was significantly associated with higher relapse rates. Multivariable analyses confirmed that residual IDH1 mutations lacked independent prognostic significance. The findings indicate that isolated detection of IDH1 mutations before allogeneic HCT should not be considered evidence of clinically meaningful MRD, highlighting that not all AML-associated mutations have equivalent prognostic value in remission.
- LK21-01d Dillon LW, Gui G, Ravindra N, et al. Measurable residual FLT3 internal tandem duplication before allogeneic transplant for acute myeloid leukemia. JAMA Oncology. 2024 Aug 1; 10(8):1104-1110. doi:10.1001/jamaoncol.2024.0985. Epub 2024 May 2. PMC11066770.
- This cohort study evaluated the prognostic impact of MRD from FLT3-ITD detected before allogeneic HCT in adults with AML in first complete remission. Using centralized, ultra-sensitive DNA sequencing of pre-transplant blood from 537 patients, the study found that any detectable residual FLT3-ITD was associated with a significantly higher relapse risk and worse overall survival after transplant. Risk increased in a dose-dependent manner, with the highest relapse rates observed in patients with variant allele frequency (VAF) ≥0.01%. Conditioning intensity modified risk: Myeloablative or melphalan-containing regimens reduced relapse compared with reduced-intensity or nonmyeloablative approaches, particularly in MRD-positive patients. The findings establish pre-transplant FLT3-ITD MRD as a strong, quantitative predictor of post-transplant outcomes and support its use for risk stratification and treatment planning before allogeneic HCT.
- LK21-01e Hegde PS, Andrew G, Gui G, et al. Measurable residual FLT3 tyrosine kinase domain mutations before allogeneic transplant for acute myeloid leukemia. Bone Marrow Transplantation. 2025 Feb 1; 60(2):175-177. doi:10.1038/s41409-024-02444-7. Epub 2024 Oct 18. PMC11810768.
- This article investigated the role of FLT3-TKD mutations as a potential target for MRD testing in the pre-HCT setting for patients with AML. Using single-amplicon, ultra-deep, error-corrected next-generation sequencing (SA-NGS), 14 of 342 (4.1%) evaluable patients had positivity for FLT3-TKD mutations at a VAF of 0.1%. By univariate analysis, FLT3-TKD MRD positivity was associated with an increased 3-year cumulative incidence of relapse (HR 6.16, 95% CI 3.00-12.65, p<0.001) and decreased 3-year overall survival (HR 1.48, 95% CI 0.75-2.92, p=0.264). Using the highly sensitive SA-NGS approach, a total of 34 of 342 (9.9%) had MRD-positivity at a VAF ≥ 0.01%. However, patients with MRD-positivity at a VAF of 0.01% to 0.1% had no statistical difference in cumulative incidence of relapse or overall survival. These results suggest that, although FLT3-TKD mutations may be a useful MRD biomarker, technical limitations and VAF cutoffs must be carefully considered.
- LK21-01f Gui G, Ravindra N, Hegde PS, et al. Measurable residual mutated IDH2 before allogeneic transplant for acute myeloid leukemia. Bone Marrow Transplantation. 2025 Feb 1; 60(2):144-153. doi:10.1038/s41409-024-02449-2. Epub 2024 Oct 25. PMC11810785.
- This study investigated the role of IDH2 mutations as a potential target for MRD testing in the pre-HCT setting for patients with AML using the highly sensitive SA-NGS approach. MRD-positivity for IDH2 at a VAF of 0.1% was seen in 130 of 257 (51%) of patients and was associated with an increased 3-year risk of relapse (29% vs. 18%, 95% CI 0.2% to 22%, p=0.03) and decreased 3-year overall survival (58% vs. 83%, 95% CI -13% to -38%, p<0.001%). This remained significant in a multivariate analysis including other relevant transplant-related variables (HR 2.5, 95% CI 1.5-4.2, p<0.001). Interestingly, there was a strong interaction with other co-mutations (NPM1 and FLT3), and it was possibly associated with higher non-relapse mortality post-HCT. These findings establish a role for IDH2 as a MRD target, with caveats that co-mutations and other patient-specific factors must be accounted for.
The Leukemia Working Committee drives momentum with numerous exciting studies now underway:
- CK16-01B Identification of germline predisposition mutations in young MDS patients
- LK20-01 Acute myeloid leukemia with chromosome 17 abnormalities with or without TP53 abnormalities and outcomes after hematopoietic stem cell transplantation
- LK20-03 Evaluating outcomes of allogeneic hematopoietic cell transplantation in T-cell acute lymphoblastic leukemia
- CK22-01 Impact of somatic mutations on outcomes after allogeneic HCT in patients with MDS with ring sideroblasts (MDS-RS) and MDS / MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T)
- LK22-01 Impact of pre-allogeneic hematopoietic cell transplantation therapy in acute myeloid leukemia and myelodysplastic syndrome on post-transplant outcomes
- CK22-02 Toxicity and survival of AML / MDS patients receiving allogeneic HCT using RIC: A propensity score analysis
- CK23-01 Identifying the optimal GVHD regimen in allogeneic HCT for myelofibrosis
- LK23-01a The impact of allogeneic stem cell transplantation on acute myeloid leukemia and myelodysplastic syndrome with chromosome 3 abnormalities
- LK23-02 Prognostic impact of cytogenetic and molecular risk classification in AML after hematopoietic stem cell transplant in adolescents and young adults
- CK23-02 The mutational landscape in myelodysplastic syndrome arising from aplastic anemia and its impact on allogeneic stem cell transplantation outcomes
- LK23-03 Impact of donor source in second allogeneic hematopoietic cell transplant in patients with acute leukemia/MDS who relapsed after prior allograft during the current era (2014-2020)
- CK24-01 Identifying the optimal stem cell dosing for PBSC HCT with post-transplant cyclophosphamide
- LK24-01a Safety and efficacy of CAR-T cell therapy in relapsed/refractory acute lymphoblastic leukemia with central nervous system involvement
- LK24-01b Sequencing of chimeric antigen receptor T-cell therapy and allogeneic transplantation in adult patients with B-cell acute lymphoblastic leukemia
- LK24-01c Real world experience (RWE) of adult patients receiving CD19 CAR-T cells for B cell acute lymphoblastic leukemia (B-ALL): A CIBMTR analysis
- CK24-02 Outcomes of allogeneic HCT in patients with DDX41-mutated MDS and AML
- CK24-03 Comparison of RIC regimens for haploidentical donor HCT with post-transplant cyclophosphamide in patients with AML or MDS
- CK24-04 Comparison of post-transplant cyclophosphamide-based RIC regimens for older patients with AML and MDS
- LK25-01 Comparison of FluFTBI and other myeloablative conditioning regimens for haploidentical and mismatched unrelated hematopoietic cell transplant with post-transplant cyclophosphamide in patients with acute leukemia
- LK25-02 Myelodysplastic neoplasms with hypoplasia (MDS-h) or fibrosis (MDS-f): Distinct clinical entities compared to other MDS subtypes
- LK25-03 Impact of post-transplant cyclophosphamide based GVHD prophylaxis on outcomes in patients with CMML undergoing allogeneic stem cell transplant
The Leukemia Working Committee invites new participants and proposals. We promote collaboration across committees and encourage using external datasets to better define transplantation’s role and timing as new non-transplant strategies emerge. We advance HCT / ACT in a rapidly evolving therapeutic landscape and provide young investigators with opportunities to work with novel study designs and statistical methods for observational research. Join us at our next in-person meeting during the Tandem Meetings in Salt Lake City, UT, in February 2026. We’re proud of the projects that have advanced the field, and we look forward to reviewing new proposals to continue improving patient outcomes.
Explore planned, in-progress, and completed studies—plus their published results— on the Leukemia Working Committee webpage.
Morbidity, Recovery and Survivorship Working Committee
Committee Leadership
 |
 |
 |
|
|
Sairah Ahmed, Co-Chair |
Seth Rotz, Co-Chair |
Annie Im, Co-Chair |
|
 |
 |
 |
|
|
Amy Moskop, Scientific Director |
Rachel Phelan, Scientific Director |
Ruta Brazauskas, Statistical Director |
|
 |
 |
 |
|
|
Soyoung Kim, Statistical Director |
Andrew Peterson, Statistician |
Poorva Bindal, Page Scholar |
|
 |
 |
 |
|
|
Reena Jayani Kosarzycki, Page Scholar |
Brandon Nuechterlein, Consumer Advocacy Member |
Rebecca Higgins, Consumer Advocacy Member |
The Morbidity, Recovery and Survivorship Working Committee provides scientific oversight of studies related to the post-HCT / ACT trajectory, except for GVHD toxicities. This ranges from consequences of using particular preparative regimens, prevention strategies, or supportive care to issues related to long-term survivorship, such as organ toxicity and psychosocial effects on health-related quality of life. We take advantage of CIBMTR’s extensive clinical database to study these early complications and late effects of treatment, which include characterizing specific toxicities as well as understanding which survivors are at greatest risk for developing these complications. A better understanding of HCT- and ACT-related early toxicities and late effects is important not only for improving supportive care and surveillance in clinical practice but also for the development of new strategies associated with low rates of undesirable complications of HCT and ACT, resulting in improvements in recovery and subsequent survivorship. Such studies also allow us to give our patients and their loved ones realistic prospects regarding their recovery after undergoing HCT / ACT.
The Morbidity, Recovery and Survivorship Working Committee meets annually in person at the Tandem Meetings. The Co-Chairs, CIBMTR Scientific Directors, Consumer Advocacy Committee members, and statisticians meet monthly by teleconference to ensure the timely completion of projects, reassess priority areas, and promote and develop the scientific agenda. We look forward to the 2026 committee meeting in Salt Lake City, UT, and all the discussion and collaboration that is to come.
A list of the committee’s studies, including recent publications, is provided on the committee’s webpage. Our recent research focus areas and achievements include:
- Identifying cardiovascular risk factors in survivors of childhood HCT in a collaborative study with the Childhood Cancer Survivor Study
- Determining the impact of comorbidities on conditioning intensity selection
- Comparing lymphodepleting chemotherapy prior to CAR-T for lymphoma
- Highlighting outcomes in patients receiving HCT and solid organ transplantation in two manuscripts published in collaboration with the Organ Procurement and Transplantation Network
Our extensive portfolio also comprises multiple protocols in development, analysis, and manuscripts in preparation. These cover a wide range of topics related to the care of HCT and ACT recipients. We are grateful for the ongoing enthusiasm and engagement of the participants in the Morbidity, Recovery and Survivorship Working Committee.
Our committee also remains involved in projects that lead to published reviews targeting areas of post-transplant late effects. Building on our strong history of international collaboration, we worked with our EBMT colleagues to develop a process calling for proposals, choosing a pertinent late effects topic, and then conducting a formal systematic review on the chosen topic. Currently, a systematic review focused on female-specific late effects is starting, and we are excited to continue this effort moving forward. We are thankful for these opportunities to engage more junior investigators within our committee efforts!
The Morbidity, Recovery and Survivorship Committee’s success depends on new ideas, testable hypotheses, and the participation of individuals with different perspectives and scientific backgrounds. We seek novel ideas and encourage the involvement of junior investigators interested in outcomes research. We believe early involvement leads to the long-term participation of junior investigators in committee activities.
Before you propose a study, please review the data forms available online to determine if the data are available to answer your question. Since some of the proposed studies may require data across many decades, it is possible that the type and way the data were collected also changed with time. We look forward to the growth of our PRO data, which we hope can be used in future committee studies. We also encourage the community to review the publicly available datasets as a way to use data outside the standard working committee process. Please reach out early if there are any questions regarding data availability.
To learn more about the committee or to discuss your research ideas and proposals, we encourage you to contact one of the members of the committee leadership team (listed above). We look forward to your participation in upcoming studies and at future Tandem Meetings!
CIBMTR Page Scholars
Over the coming months, we will continue to highlight each of the Page Scholars, introducing them to our CIBMTR Community by sharing who they are, their goals, and their interest in the Page Scholar Program.
 |
Ryan Stubbins, MD, MS – Leukemia Working Committee I am a hematologist and transplant / cell therapy physician in the leukemia / BMT program of British Columbia. I completed medical school, residency training, a Master of Science, and hematology / BMT training at the Universities of Saskatchewan, Alberta, and British Columbia. I also pursued additional research training at the British Columbia Cancer Genome Sciences Center and the University of Chicago. My clinical and research interests focus on myeloid malignancies, rare hematologic diseases, and transplantation / cell therapy. I am incredibly grateful for the opportunity to work with CIBMTR as a Page Scholar. Through this program, my goal is to develop a deep understanding of how the CIBMTR registry operates and learn what makes it so successful in producing practice-changing, real-world studies. The Page Scholar Program offers a unique opportunity to collaborate with one of the leading organizations in our field, and I hope to leverage this experience to contribute to research that improves the outcomes for patients undergoing transplantation and cellular therapies. “Ryan’s engagement and input demonstrate his genuine dedication to the program. He continues to lead an ongoing project and is starting a second study in collaboration with EBMT.” |
|
 |
Arpita Ghandi, MD, MS – GHVD Working Committee I serve as an assistant professor in the Department of Blood and Marrow Transplant at the Knight Cancer Institute, Oregon Health & Science University. I focus clinically on allogeneic transplantation for myeloid malignancies, with a particular interest in patients with myeloproliferative neoplasms. My research aims to improve access to allogeneic HCT, develop novel strategies for GVHD prevention, and expand availability of standard-of-care therapies and clinical trials. The Page Scholars Program has provided valuable opportunities to deepen my understanding of CIBMTR operations. Through weekly statistics meetings, I gain meaningful insights into data collection processes and statistical methodologies. As a member of the GVHD Working Committee, I help review abstracts and develop submissions based on existing CIBMTR studies. I look forward to continuing to collaborate with committee leadership and the statistics team as we evaluate study proposals and refine research design. Engaging with leaders in the field and building new mentorships has been especially rewarding and aligns closely with my goals for ongoing academic and professional development. “Arpita integrates seamlessly into the GVHD Working Committee, actively advancing proposals and providing critical feedback. She eagerly takes on tasks and remains highly engaged in all working committee activities.” |
|
 |
Reena Jayani-Kosarzycki, MD – Morbidity, Recovery and Survivorship Working Committee I work with the Morbidity, Recovery, and Survivorship Working Committee. I am an HCT physician at Vanderbilt University Medical Center with a research and clinical focus in older and frail adults undergoing HCT or cellular therapy. I am honored to serve as a Page Scholar and look forward to collaborating with and learning from leaders in the field to leverage the strengths of the registry, overcome its limitations, and cultivate skills in problem-solving throughout the research process—from study design and analysis to final publication. “Reena is a wonderful addition to our committee. She leads an ongoing CIBMTR study that is nearing completion and consistently provides thoughtful feedback on new proposal submissions. We are thrilled to have her on the committee!” |
Get Ready for the 2026 Tandem Meetings – Register Today!
By Alicia Halfmann and Maira Brey
Join us at the 2026 Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR, taking place February 4-7, 2026, in Salt Lake City, UT!
Register now to secure your spot. The Tandem Meetings deliver cutting-edge science, unparalleled networking, and world-class education for HCT, cellular therapy, and gene therapy communities. Don’t miss the premier event in the field!
Digital Access Option
Can’t join us in person? No problem! Choose Digital Access and experience the scientific program from anywhere. With Digital Access registration, you get to:
- Watch on-demand session recordings just hours after the live session ends, including scientific plenary and concurrent sessions, oral abstract sessions, honorific lectures, symposia, and educational tracks.
- Maintain access to most on-demand session recordings for at least 30 days after the meetings conclude.
- Earn continuing medical, nursing, and pharmacy credits by viewing on-demand session recordings and completing evaluations by Tuesday, March 10, 2026.
Register Now: 2026 Tandem Meetings Registration
Why Attend?
- Explore a comprehensive scientific program with plenary sessions, oral abstracts, and specialized tracks covering the latest in transplantation and cellular therapy.
- Celebrate excellence through honorific lectures and awards, including the Mortimer M. Bortin and E. Donnall Thomas Lectures, plus distinguished service and lifetime achievement honors.
- Connect with colleagues and leaders in person or online through dynamic networking opportunities.
- Earn CME credits by completing session evaluations and the overall meetings evaluation to receive your CME Credit Certificate. For details on additional credit types and evaluations, visit the Evaluations and Credits page in the online program or mobile app.
Mobile App
Download the official Tandem Meetings mobile app for instant access to schedules, maps, and interactive features. Build your personal agenda, message other attendees, and join live polls. Search TANDEM 2026 in your Apple App Store or Google Play to download the app today.
Join the Conversation: #Tandem26
Share your experience on social media using #Tandem26.
Don’t wait—secure your spot today and experience the Tandem Meetings your way!
Tandem Meetings News
Follow detailed coverage of sessions and presenters before, during, and after the 2026 Tandem Meetings.
Research Dissemination: What It Is and Why It Matters
Research dissemination is the process of sharing important findings from scientific studies in ways that make them accessible and useful to those who can benefit most, including healthcare professionals, patients, caregivers, and policymakers. Publishing results in academic journals and presenting at conferences are essential steps, but additional steps are needed. To truly impact patient care and outcomes, we must move beyond academic circles and communicate research in formats that are clear, understandable, and actionable for all relevant audiences.
Successful dissemination tailors messages to each audience instead of relying on a one-size-fits-all approach. It presents complex research results in clear, relevant formats—whether delivering detailed data for clinicians or practical, easy-to-understand information for patients.
Example: CIBMTR’s Approach
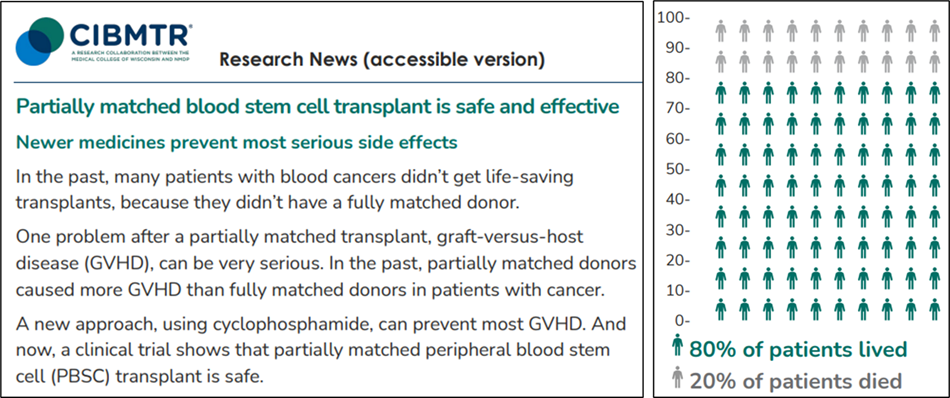
CIBMTR ensures that practice-changing evidence from transplant and cell therapy research reaches the right people. For example, when results from the ACCESS clinical trial were published, CIBMTR created both a patient-friendly summary and a clinician-focused report. These resources helped patients understand the safety and effectiveness of new treatments and provided clinicians with the detailed data they need for medical decision-making.
Patient-friendly summary of ACCESS trial results
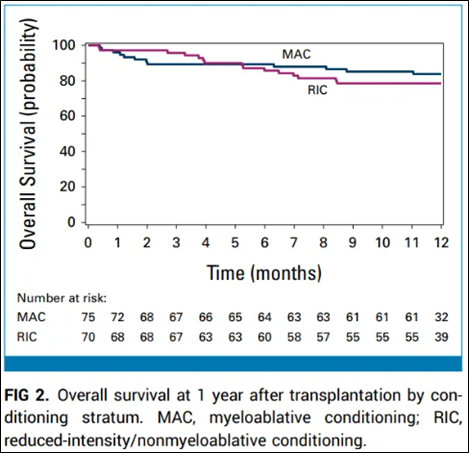
Clinician-focused report of ACCESS trial results
Key Takeaway
Organizations like CIBMTR accelerate the adoption of new practices by tailoring how they share research findings and moving beyond traditional academic channels. These efforts ultimately improve outcomes for patients with blood cancers and other serious conditions.
BMT CTN Spotlight
By Heather Miller, MHA
The BMT CTN is in its fifth grant cycle and has now enrolled more than 17,250 patients. Established in 2001, the Network is funded by the NHLBI and NCI.
Clinical Trials: Open Enrollment
The BMT CTN encourages widespread transplant community participation in clinical trials. If your center is interested in participating, please visit the BMT CTN website.
There are 7 active BMT CTN trials. Of the BMT CTN-led trials, 2 protocols are open to accrual, and 5 are in development. The following BMT CTN protocols are open to accrual:
- BMT CTN 2203 – A randomized, multicenter, Phase III trial of tacrolimus / methotrexate / ruxolitinib versus post-transplant cyclophosphamide / tacrolimus / mycophenolate mofetil in non-myeloablative / reduced intensity conditioning allogeneic peripheral blood stem cell transplantation
- Enrollment is currently paused for the 50-patient run-in analysis. Phase III enrollment is expected to resume in the second quarter of 2026.
- BMT CTN 2207 – A Phase II trial of non-myeloablative conditioning and transplantation of haploidentical related, partially HLA-mismatched, or matched unrelated bone marrow for newly diagnosed patients with severe aplastic anemia.
- This trial also offers a proactive financial navigation service as part of a BMT CTN Access to Clinical Trials initiative.
If your center is participating in BMT CTN 2203 or BMT CTN 2207, please consider participating in BMT CTN 2302 - Facilitating Activation of Study Trials (FAST), which is a time-and-motion study to understand the infrastructure, processes, barriers, and effective and ineffective center practices related to activation of a cooperative group trial.
BMT CTN Publications
There are 212 BMT CTN published articles, including 49 primary analyses. Researchers published 4 primary manuscripts in 2025:
- 1507 Adult Cohort: Kassim AA, Walters MC, Eapen M, et al. Haploidentical bone marrow transplantation for sickle cell disease. NEJM Evidence. 2025 Mar 1; 4(3):EVIDoa2400192. doi: 10.1056/EVIDoa2400192. Epub 2025 Feb 25. PMC11932095. https://pubmed.ncbi.nlm.nih.gov/39998298/
- 1704: Sorror ML, Saber W, Logan B, et al. Novel composite health assessment risk model for older allogeneic transplant recipients: BMT-CTN 1704. Blood Advances. 2025 Jul 8; 9(13):3268-3280. doi: 10.1182/bloodadvances.2025015793. PMC12246602. https://pubmed.ncbi.nlm.nih.gov/40101246/
- 1201: Andreadis C, Bobek O, Hsi ED, et al. Ibrutinib added to standard conditioning and as maintenance therapy following autologous hematopoietic stem cell transplantation for relapsed or refractory activated-B-cell type diffuse large B-cell lymphoma: Primary analysis of the US intergroup double-blind randomized Phase III study Alliance A051301/BMT-CTN 1201. Leukemia & Lymphoma. 2025 Oct 1; 66(10):1903-1912. doi: 10.1080/10428194.2025.2525982. Epub 2025 Jul 9. PMC12321088. https://pubmed.ncbi.nlm.nih.gov/40632607/
- 1702: Lee SJ, Logan B, Horowitz MM, et al. Primary results from Blood and Marrow Transplant Clinical Trials Network 1702: Clinical transplant-related long-term outcomes of alternative donor allogeneic transplantation. Journal of Clinical Oncology. 2025 Nov 1; 43(31):3369-3380. doi: 10.1200/JCO-25-00206. Epub 2025 Sep 18. PMC12700293. https://pubmed.ncbi.nlm.nih.gov/40966481/
About the BMT CTN
CIBMTR shares administration of the BMT CTN Data and Coordinating Center with NMDP and The Emmes Company. Together, these three organizations support all BMT CTN activities. The BMT CTN Steering Committee is currently under the leadership of John Levine, MD, (Mount Sinai) as Steering Committee Chair; Stephanie Lee, MD, (Fred Hutchinson Cancer Center) as Steering Committee Chair-Elect; and Miguel-Angel Perales, MD, (Memorial Sloan Kettering Cancer Center) as Steering Committee Vice-Chair.
To get up-to-date information about BMT CTN studies, meetings, and news, be sure to follow us on X (Previously known as Twitter): @BMTCTN
ACT Corner
By Amy Moskop, MD, MS
Adoptive Cellular Therapies Stakeholders Council
As the incoming ACT lead, I look forward to working with the ACT Stakeholders Council to continue to expand our ACT research portfolio and further streamline data collection and collaboration across our contributing centers.
In 2026, the primary goal of CIBMTR ACT is to continue to expand and optimize the database to capture data on new cellular therapy products and broaden indications for cell and gene therapy. We will pursue initiatives to grow the registry for solid tumor cellular therapy. Currently, CIBMTR solid tumor and response forms apply to synovial sarcoma, but as the field advances, we will leverage this infrastructure to include additional diseases and products. With the increasing number of patients treated with commercial gene therapy products, the ACT Stakeholders Council will identify and address gaps in data collection, such as fertility, within the registry. We will continue discussing and planning these initiatives at our in-person meeting during the Tandem Meetings in February 2026.
We recently welcomed new members:
- Frederick Locke, MD, Chair
- Christine Duncan, MD, Member
- Yi Lin, MD, PhD, Member
CIBMTR ACT Stakeholders Council Members:
- Frederick Locke, MD, Chair (Moffitt Cancer Center, Tampa, FL)
- Dimas Padilla, CAC Representative (Bethune-Cookman University, Daytona Beach, FL)
- Rayne Rouce, MD, Member (Baylor College of Medicine Center for Cell and Gene Therapy, Houston, TX)
- Christine Duncan, MD, Member (Dana-Farber Cancer Institute, Boston, MA)
- Yi Lin, MD, PhD, Member (Mayo Clinic, Rochester, MN)
- Lori Henderson, PhD, Ex Officio Member (NIH-NCI Government Agency Partners, Bethesda, MD)
- Amy Moskop, MD, MS, Ex Officio Member (CIBMTR MCW, Milwaukee, WI)
- Dawn Lyons, Ex Officio Member (CIBMTR MCW, Milwaukee, WI)
Adoptive Cellular Therapies Data Collection
In January 2026, CIBMTR will release the revised Cellular Therapy Essential Data forms (version 10), designed to capture new therapies and novel toxicities as the cellular immunotherapy field evolves. The ASTCT CAR T-Cell Toxicity Group reconvened to update its original recommendations on CRS grading and immune effector cell-associated neurotoxicity syndrome (ICANS), and will provide clearer definitions for both classic and emerging CAR-T toxicities. Our CIBMTR ACT team collaborated with a panel of data managers and cell therapy experts to integrate these updates into the revised forms. Key enhancements include detailed treatment dates for CRS, the addition of immune effector cell-associated HLH-like syndrome (IEC-HS) aligned with recent diagnostic criteria and grading recommendations, and an updated neurotoxicity section featuring tumor inflammation-associated neurotoxicity (TIAN). We will seek community feedback on these improvements.
CIBMTR is partnering with 2 pharmaceutical companies to leverage our infrastructure for regulatory long-term follow-up of patients receiving commercial gene therapy products. We are also collaborating with the Centers for Medicare and Medicaid Innovation (CMMI) on a study to meet CMMI Access Model data requirements for patients with sickle cell disease. We are developing innovative data-linking and sharing approaches and expanding ePRO data collection, including for pediatric patients. As part of these initiatives, we continue to enhance data collection to support research, including updates to the sickle cell disease forms in October 2025.
Center-Specific Survival Analysis and Center Outcomes Forum
By Carol Doleysh
The SCTOD is part of the US HRSA-funded C.W. Bill Young Cell Transplantation Program that collects data on all allogeneic HCT performed in the US and on transplants done elsewhere using cellular products that originated in the US.
Center-Specific Survival Analysis
Outcomes reporting in allogeneic HCT provides essential information for patients, payers, and government agencies and ensures compliance with current laws. Under the SCTOD contract, CIBMTR analyzes one-year survival rates at every US transplant center annually and generates a report designed as a quality improvement tool for centers. To be included in the analysis, transplant centers must maintain at least one year of follow-up on more than 90% of related and unrelated HCT recipients within the reporting period.
CIBMTR distributed the 2025 Center-Specific Survival Analysis (CSA) Report in mid-December to center directors, payers, and FACT. The report includes first allogeneic HCT performed in the US between 2021 and 2023. We also provided an executive summary highlighting key elements and analysis results. Updated data are available on the NMDP website, and transplant centers can access quality improvement tools—Center Performance Analytics and the Survival Calculator—through the secure CIBMTR Portal. Submit any questions regarding CIBMTR Portal account credentials via CIBMTR Center Support (https://nmdp.service-now.com/csm) by selecting CIBMTR Center Maintenance, then CIBMTR Portal Help. Visit the dedicated CSA page (https://cibmtr.org/CIBMTR/Resources/Center-Specific-Survival-Analysis) for information on CSA methodology and Center Outcomes Forums, frequently asked questions, and links to the CIBMTR Portal and NMDP Transplant Center Directory.
A recommendation from the 2023 Center Outcomes Forum was to hold an informational session during the Tandem Meetings to share key developments about the CSA, including updated risk adjustment, analysis findings, and future plans. This year, the CIBMTR session during the Tandem Meetings will highlight how research informs the CSA and how CIBMTR collaborates with researchers to study the CSA’s impact. Join us on February 4, 2026, for this important opportunity to learn more about the CSA firsthand—especially for center medical directors, administrative directors, and data professionals.
Center Outcomes Forum
CIBMTR hosted the Center Outcomes Forum on November 20, 2025, to discuss its approach to the CSA and gather meaningful recommendations for improvement. We remain committed to continuously improving this high-impact report. The 2025 Forum addressed key topics, including recommendations for MRD for ALL and AML, optimizing handling of comorbidities in adults and pediatrics, and refining transplant-related factors included in risk adjustment. Invitees included transplant physicians, center directors, representatives from ASTCT and its Committee on Quality Outcomes, FACT, government funding agencies, patients, private payers, and statisticians. Explore details of all previous Center Outcomes Forums, including agendas and summaries, on the CIBMTR website (https://cibmtr.org/CIBMTR/Meetings/Materials-Archive/Center-Outcomes-Forum).
PRO Corner
CIBMTR PRO Team Presents at ISOQOL Conference
In October, CIBMTR’s PRO team presented 4 abstracts and 2 symposia at the 2025 International Society for Quality-of-Life Research (ISOQOL) conference in Milwaukee. Rachel Cusatis, PhD, senior scientific director of patient-centered research and survivorship, showcased how CIBMTR leverages its clinical registry infrastructure to build PRO collection and deepen insights into patient experiences. Her presentation, part of an international symposium, featured expert perspectives from colleagues in England and Italy. Dr. Cusatis also led a CIBMTR-sponsored symposium with Deborah Mattila, survey research group manager, and Miranda Kapfhammer, clinical research coordinator, highlighting successes and challenges of the PRO Protocol, a centralized PRO data collection resource that supplements traditional cellular therapy outcomes like survival and relapse. Idayat Akinola, clinical research coordinator, presented 5-year enrollment trends for the PRO Protocol, while Carlos Litovich, post-doctoral research fellow, discussed disparities affecting representation in cellular therapy research. Oyiza Usman, PhD student and research assistant, presented on financial toxicity in the US population, and Miranda Kapfhammer shared an evaluation of PROMIS and EORTC cognitive function measures. Sarah Thryselius-Reed, research assistant, contributed a poster reviewing methods for assessing financial challenges among cellular therapy patients. The PRO ream was proud to showcase CIBMTR’s impactful work and connect with global leaders advancing quality-of-life research at ISOQOL 2025! Below are photos of the team in action.
PRO Protocol Participation Expands
CIBMTR is excited to announce that the PRO Protocol is now enrolling pediatric participants aged 12 and older. This expansion significantly broadens the reach and representativeness of patient-reported data among pediatric recipients of cellular and gene therapies. By including younger patients, we capture their unique perspectives on recovery, quality of life, and survivorship, areas in which patient voice is essential yet historically underrepresented.
CIBMTR will continue obtaining PRO-specific consent exclusively through direct outreach to participants. All survey instruments have been reviewed for age-appropriate usability, and resources, including an informational letter, are available to support sites that want CIBMTR to enroll their pediatric patients. If your site is interested, contact us at surveys@cibmtr.org.
For more information, please visit our webpage.
Cracking the Genomic Code to Prevent Relapse in MDS Patients
For patients with MDS, a stem cell transplant is often the only chance for a cure. However, for nearly 40% of patients, that hope is undermined by disease relapse after transplant, a devastating outcome. Why relapse occurs, and whether it can be predicted, remain critical unanswered questions.
In the latest issue of JCO Precision Oncology (https://ascopubs.org/doi/10.1200/PO-25-00140), our bioinformatics research team, together with researchers across CIBMTR, shares an innovative genomics study that could shed new light on this important question. By analyzing whole-genome sequencing data from nearly 500 patients with MDS who underwent transplantation, we identified 10 novel genetic markers linked to relapse risk. These included alterations in 2 genes (HNRNPA3 and TENM2), rare inherited variants in HSPC324 gene, and a large structural DNA deletion. When incorporated into existing clinical risk models, these genomic markers markedly improved prediction accuracy, increasing the concordance index from 0.547 with clinical factors alone to 0.747. Importantly, the greatest gains were seen among patients who previously fell into “gray areas” under standard risk scoring systems.
These findings point to a future in which transplant decisions and post-transplant monitoring can be tailored to each patient’s unique genetic profile. Although further validation in larger and more diverse populations is needed, this study represents a meaningful step toward more precise, personalized care for patients with MDS.
Reference
Zhang T, Auer PL, Dong J, et al. Novel genomic biomarkers improve post-hematopoietic cell transplantation relapse risk stratification for patients with myelodysplastic syndromes. JCO Precision Oncology. 2026 Jan:10:e2500140. doi: 10.1200/PO-25-00140. Epub 2026 Jan 23. PMID: 41576307.
Publicly Available Datasets
 In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, CIBMTR makes the final datasets from published studies publicly available on CIBMTR’s Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, CIBMTR makes the final datasets from published studies publicly available on CIBMTR’s Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
While providing these data, CIBMTR is committed to safeguarding the privacy of participants and protecting confidential and proprietary data. Upon accessing the datasets page on CIBMTR’s public website, the viewer is notified that the dataset was collected by CIBMTR, and CIBMTR’s supporters are listed. The webpage also clearly notes the terms and conditions of dataset usage.
NEW datasets are now available online.
Share Research in Plain Language
By Jennifer Motl
These new plain-language summaries of CIBMTR research may help your patients:
|
|
People who get an organ transplant and then a blood or marrow transplant may have serious problems; read more. |
|
|
Six main barriers prevent patients from getting transplant for multiple myeloma; read more. |
Find more summaries at:
Our Supporters
CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; and 75R60222C00008, 75R60222C00009, and 75R60222C00011 from the Health Resources and Services Administration (HRSA).
Additional federal support is provided by OT3HL147741, P01CA111412, R01CA100019, R01CA218285, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01FD008187, R01HL171117, R21AG077024, U01AI069197, U01AI184132, U24HL157560, and UG1HL174426.
Support is also provided by Australian Bone Marrow Donor Registry; Boston Children’s Hospital; Fred Hutchinson Cancer Center; Gateway for Cancer Research, Inc.; Jeff Gordon Children’s Foundation; Medical College of Wisconsin; NMDP; Patient Center Outcomes Research Institute; PBMTF; St. Baldricks’s Foundation; Stanford University; Stichting European Myeloma Network (EMN); and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune LLC; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc.; Amgen, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; Autolus Limited; Beam; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, inc.; Blueprint Medicines; Bristol Myers Squibb Co.; CareDx Inc.; CSL Behring; CytoSen Therapeutics, Inc.; DKMS; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Genetix; Gift of Life Biologics; Gift of Life Marrow Registry; HistoGenetics; ImmunoFree; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Japan Hematopoietic Cell Transplantation Data Center; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin International plc; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Medexus; Merck & Co.; Mesoblast, Inc.; Millennium, the Takeda Oncology Co.; Miller Pharmacal Group, Inc.; Miltenyi Biotec, Inc.; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc.; OriGen BioMedical; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pierre Fabre Pharmaceuticals; PPD Development, LP; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc.; Servier; Sobi, Inc.; Sociedade Brasileira de Terapia Celular e Transplante de Medula Óssea (SBTMO); Stemcell Technologies; Stemline Technologies; STEMSOFT; Syndax; Takeda Pharmaceuticals; Talaris Therapeutics; Tscan Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.














