November 2022 Newsletter

Volume 28, Issue 4
Table of Contents:
-
Perspectives
-
Plasma Cell Disorders Working Committee
-
Kwang Woo Ahn, PhD, Appointed Associate Chief Statistical Director
-
Cellular Immunotherapy for Cancer Working Committee
-
New Combined Working Committee
-
2023 Tandem Meetings
-
Bioinformatics Research Tools Benefit Users Around the World
-
Health Services Research Program
-
CIDR: Beyond the Finish Line
-
Stem Cell Therapeutic Outcomes Database
-
PRO Data Now Available in DBtC
-
MEASURE Now Enrolling
-
Sharing Your Research
-
Publicly Available Datasets
-
Our Supporters
-
Abbreviations
Perspectives: Why are we so stubborn?
By John Wingard, MD

I have a shirt that I really love. That surprises me since I am totally not into clothes, and I hate shopping for myself. The shirt is plaid and contains my college's colors in subtle ways. It fits me well. I can wear it to work, parties, and sports events. Colleagues, friends, casual acquaintances, and even my patients compliment me on my shirt. Total strangers stop me on the street and ask me where I got it so they can get one just like mine. I feel good when I wear the shirt.
There is only one problem with my shirt: It is so old, the collar and sleeves are frayed. My wife keeps saying: "John, you need to throw it away." Of course, I ignore her advice and hide it in the back of the closet so she does not throw it away herself. I understand her point, but I have my reasons.
I thought about my shirt when we planned the ASTCT / CIBMTR / EBMT Plenary Session at the 2022 Tandem Meetings addressing "The Challenge of Translating Evidence into Action in HCT," and I thought about it even more during the August BMT CTN Steering Committee meeting.
We spend a lot of time and money designing and conducting trials to test new interventions to improve outcomes and change practice. Yet, sometimes successful trials do not convince clinicians to adopt the new practice.
Why is this? Sometimes, there are valid reasons to resist change: It takes too much time to adopt the new thing, it is too hard to get everyone in my center to agree, the data are not believable, the findings are in patients that are very different from my patients, or it is too expensive. One of the often-cited problems is that the rigid eligibility criteria for trials exclude most of the patients we care for, and we are not sure how generalizable the findings are. Maybe, we changed our minds that the new intervention is not as great as we thought.
Even when we agree that the new intervention is better, change is still hard. Studies across all medical disciplines show this to be true. Some centers are better at adopting new practices than others. It is good to know that there are enablers to change: Your institution may be supportive of innovation, payers may insist on change, accreditation bodies may expect the adoption of "standards," and patients may expect you to adopt "breakthroughs" that they have heard about through their lay sources of information. A local champion to push a new practice forward can often make all the difference. Certainly, the local culture of a transplant team is crucial in adopting new practices.
Kudos to Linda Burns, MD, and Nandita Khera, MD, MPH, who are chairing a BMT CTN task force to try to improve the translation of evidence into practice in BMT. The task force recommended developing a committee to incorporate dissemination and implementation considerations into each BMT CTN trial. They described the importance of taking action even before the trial, the value of identifying the key stakeholders, the need to identify local champions, and the importance of identifying the impact of change on patients and non-transplant providers.
The coin of the realm for evaluating the quality of research is its impact. It would be a shame to do a trial that shows something that truly works, and we are slow or uneven to adopt it or do not adopt it at all. As Drs. Burns and Khera emphasized, it is time for us to consider implementation when we are designing trials.
Perhaps, my wife is also right: It is past time to throw away my beloved shirt. I need to go shopping this weekend.
Plasma Cell Disorders Working Committee

Past and present members of the CIBMTR Plasma Cell Disorders Working Committee pose for a photo during the meeting at the 2022 Tandem Meetings. From left to right: Shaji Kumar, MD; Heather Landau, MD; Nina Shah, MD; Ruta Brazauskas, PhD; and Noel- Estrada Merly, MPH.
Current Committee Leadership
 |
 |
 |
| Nina Shah, MD, University of California San Francisco Medical Center, Chair |
Muzaffar Qazilbash, MD, MD Anderson, Chair | Heather Landau, MD, Memorial Sloan Kettering Cancer Center, Chair |
 |
 |
|
| Ruta Brazauskas, PhD, PhD Statistician | Temitope Oloyede, MD, MPH, MS Statistician |
Committee Leadership
Co-Chairs:
- Nina Shah, MD, University of California San Francisco Medical Center, San Francisco, CA
- Muzaffar Qazilbash, MD, MD Anderson Cancer Center, Houston, TX
- Heather Landau, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Scientific Director:
- TBD
Statistical Director:
-
Ruta Brazauskas, PhD, CIBMTR MCW
Statistician:
-
Temitope Oloyede, MD, MPH, CIBMTR MCW
Multiple myeloma is the most common indication for HCT in the US. The Plasma Cell Disorders Working Committee collaborates with investigators from around the world to define the optimal utilization of transplantation for not only multiple myeloma but also other plasma cell disorders, such as light chain amyloidosis, Waldenstrom macroglobulinemia, POEMS syndrome, and plasma cell leukemia.
We have five ongoing projects, including outcomes of autologous HCT in light chain deposition disease patients, differences in outcomes of myeloma treatment worldwide, secondary primary malignancies after cell transplantation for multiple myeloma patients, and bortezomib-based versus lenalidomide maintenance therapy for high-risk multiple myeloma patients. Noteworthy accomplishments from this committee in 2021-2022 include four peer-reviewed publications, two presentations at the American Society of Clinical Oncology (ASCO) Annual Meeting, one presentation at the ASH Annual Meeting, one presentation at the EBMT Annual Meeting, and one presentation at the Tandem Meetings.
In 2021, we received 50 proposals, 5 of which were presented at the 2022 Tandem Meetings; one was accepted as a study. The Plasma Cell Disorders Working Committee is always seeking interesting and novel ideas for study as well as encouraging the involvement of junior investigators and those wanting to break into the field of HCT and outcomes research. We believe early involvement encourages long-term participation of young investigators in committee activities.
Our committee provides a platform for consolidating ideas from oncologists across the country regarding the role of transplant in the management of multiple myeloma, light chain amyloidosis, Waldenstrom's macroglobulinemia, POEMS syndrome, and germ cell tumors. The CIBMTR houses the largest set of data related to transplantation outcomes for these disorders. By serving as a partner for researchers, we leverage the power of big data to undertake projects and answer questions that would be impossible for a single institution to accomplish. This is particularly relevant for the rare plasma cell disorders for which no institution would have adequate numbers to answer any critical questions related to treatment. As a byproduct of the collaborative process, stronger connections and commitment to the overall mission of the CIBMTR are achieved, and a positive feedback loop results. The vision of the committee is to keep the positive feedback loop alive and have it lead to greater national and international integration of transplant-related research that will accelerate the discovery of cures for patients afflicted by these diseases.
The success of the working committee depends on new ideas and testable hypotheses as well as the participation of individuals with diverse perspectives and scientific backgrounds. The Plasma Cell Disorders Working Committee encourages all investigators with an interest in these diseases to propose a study. Information on how to propose a study can be found on the CIBMTR's How to Propose a Study webpage.
To learn more or discuss your research ideas and proposals, contact one of the members of the working committee leadership team.
We encourage our 525 current working committee members to actively participate. We look forward to your participation next February at the 2023 Tandem Meetings.
Kwang Woo Ahn, PhD, Appointed Associate Chief Statistical Director

We are pleased to announce that Kwang Woo Ahn, PhD, has been appointed as Associate Chief Statistical Director for the CIBMTR. Dr. Ahn is a Professor in the Division of Biostatistics at MCW and has been working with various committees of the CIBMTR since joining MCW in 2008, including the Lymphoma, Pediatric Cancer, Regimen-Related Toxicity and Supportive Care, Chronic Leukemia, and Infection and Immune Reconstitution Working Committees.
In addition, he has published 119 peer-reviewed manuscripts. including 15 methodologic publications in leading statistical journals focusing on survival and competing risks data, variable selection, and high-dimensional data analysis, most of which are directly applicable to the analysis of transplant and cell therapy data. He also successfully obtained multiple research grants as PI, including a contract as PI with the FDA on "Commensurate Prior Models Accommodating Historical Controls for Clinical Trials with Matched and/or Interval-Censored Data," as well as past grants with the National Science Foundation, the Advancing a Healthier Wisconsin endowment, and the Medical College of Wisconsin Cancer Center. In his new role, he will work closely with the Chief Statistical Director, CIBMTR MCW, Mei-Jie Zhang, PhD, to provide statistical leadership to the CIBMTR.
Cellular Immunotherapy for Cancer Working Committee
Committee Leadership
Co-Chairs:
- Peiman Hematti, MD, University of Wisconsin Hospital and Clinics, Madison, WI
- Cameron Turtle, MBBS, PhD, Fred Hutchinson Cancer Research Center, Seattle, WA
- Sairah Ahmed, MD, MD Anderson Cancer Center, Houston, TX
Scientific Directors:
- Marcelo Pasquini, MD, MS, CIBMTR MCW
- Amy Moskop, MD, MS, CIBMTR MCW
Statistical Director:
-
Soyoung Kim, PhD, CIBMTR MCW
Statistician:
-
Matthew Bye, MPH, CIBMTR MCW
By Sairah Ahmed, MD
The cellular therapy registry was launched in 2016, and the Cellular Immunotherapy for Cancer Working Committee is one of the newest CIBMTR working committees and is part of the CIDR. The CIDR is a unique database that collects data about the long-term safety and efficacy CAR-T therapies. The NCI awarded the CIBMTR a grant to operate it as part of the Cancer Moonshot℠ to accelerate cancer research under the IOTN. In turn, the CIDR provides unprecedented access to these data to a diverse group of clinicians, researchers, manufacturers, payers, regulators, and the public. The CIBMTR Advisory Committee oversees the performance and activities of Cellular Immunotherapy for Cancer Working Committee, and as part of the CIDR governance structure, the activities of the working committee and study portfolio are also overseen by the CIDR Executive Committee.
I started as a co-chair of this committee this year and am honored to be able to participate in this exceptional opportunity to facilitate observational and correlative research. My journey with the CIBMTR's working committees began many years ago when I was serving as a junior faculty member in transplant and cellular therapy. The meetings were overwhelming and intimidating; however, I had the good fortune to be introduced to Mehdi Hamadani, MD, by other lymphoma-minded colleagues. He was not only a mentor but also a sponsor, and with his guidance, encouragement, and ruthless virtual red pen, I submitted several proposals and was successful in being the principal investigator for a few studies while joining many other studies. I found over the years that my experience was not uncommon; many of my colleagues as junior faculty have had the occasion to accelerate their academic careers with the support of mentors within the CIBMTR / ASTCT. As my research and clinical career unfolded, I gravitated toward non-transplant cellular therapy, and I was ecstatic when the Cellular Immunotherapy for Cancer Working Committee was established. This provided a new home for those of us with a background in transplant but also the prospect of recruiting fresh perspectives from colleagues with expertise in cellular immunotherapy who are not yet involved in the CIBMTR. I hope to be able to pay forward the mentorship and sponsorship I received not only as junior faculty but throughout my academic career as well as becoming a "friendtor - one who gives blunt constructive criticism and supports one through career leaps" as well (coined by Nina Shah, MD, who has fulfilled that role to many).
We have several exciting studies in process in the working committee and have had a number of studies presented or published in the last 12 months. We welcome the input and participation of our working committee members and can't wait for the 2023 Tandem Meetings in Orlando, Florida. We encourage all members to participate, attend, and submit proposals to the committee. This is an emerging field, and data in the registry are still maturing; however, this is an opportunity to expand the portfolio of studies and accept new proposals to develop high-impact studies.
| Chair | Study | Name | Status |
|---|---|---|---|
| Manuscript | AC17-01 | CD19 CAR T cells without HCT for AL | Manuscript prep |
| Completed | CT19-01 | ALLO vs CAR T DLBCL with prior Auto or refractory disease | Completed |
| Manuscript | CT20-01 | Comparison of commercial CAR T cells for DLBCL | Manuscript prep |
| Sairah | CT20-04 | Determinants of outcomes after CAR T cells for ALL | Data file prep |
| Peiman | AC18-01 | Effect of SCB and DLI on GVHD incidence | Manuscript prep |
| Peiman | AC16-01 | DLI After HLA-haploidentical allogeneic transplant | Manuscript prep |
| Peiman | CT20-02 | Resource utilization with CAR-T cells | Protocol development |
| Cameron | CT19-02 | Prolonged Cytopenia Following CD-19 CAR-T Therapy for DLBCL | Manuscript prep |
| Cameron | CT21-01 | Outcomes of elderly patients receiving CAR-T for DLBCL | Analyses |
| Cameron | CT20-03 | Determinants of outcomes after CAR T cells for Lymphoma | Analyses |
| Sairah | New | CD19-CAR-T therapy failure: Impact of subsequent therapy in patients with B-cell malignancies | Protocol development |
| Sairah | New | Machine learning for predicting toxicity and early clinical outcomes in DLBCL and B-ALL patients treated with commercial CAR T products in the real-world setting: an analysis of the CIBMTR registry |
Protocol development |
Submitted and Published Papers and Presentations
- Shadman M, Pasquini MC, Ahn KW, et al. Autologous transplant versus chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. doi:10.1182/blood.2021013289. Epub 2021 Sep 27. (CT19-01)
- Park J, Nikiforow S, Kim S, Hu ZH, et al. Impact of allogeneic HCT as consolidation following CD19 CAR-T cell therapy for treatment of relapsed ALL. ASH 2021. (AC 1701)
- John S, Pulsipher A, Moskop A, et al. Real-world outcomes for pediatric and young adult patients with relapsed or refractory (R/R) B-cell ALL treated with tisagenlecleucel: Update from the CIBMTR registry. ASH 2021. (SC17-08)
- Landsburg D, Firgault M, Hu ZH, et al. Real-world efficacy and safety outcomes for patients with relapsed or refractory (R/R) aggressive B-cell NHL treated with commercial tisagenlecleucel: Update from the CIBMTR Registry. ASH 2021. (SC 17-08)
- Locke F, Jacobson C, Ma L, et al. Real-world outcomes of axicabtagene ciloleucel (Axi-cel) for the treatment of large B-cell lymphoma: Impact of age and specific organ dysfunction. ASH 2021. (SC17-07)
- Jacobson C, Locke F, Hu ZH, et al. Real-world evidence of axicabtagene ciloleucel (Axi-cel) for the treatment of large B-cell lymphoma in the US. ASCO 2021. (SC 17-07)
New Combined Working Committee
We are pleased to announce the merger of two CIBMTR Working Committees: the Regimen-Related Toxicity and Supportive Care Working Committee and the Late Effects and Quality of Life Working Committee. After much discussion between the leadership of both committees and an internal voting process, the combined Working Committee will now be referred to as the Morbidity, Recovery, and Survivorship (MoReS) Working Committee. This merger will align the priorities of both Working Committees and investigators by conducting studies that focus on early as well as late complications of transplantation and give a broader framework to the joint working committee in publishing impactful studies. Rachel Phelan, MD, MPH, will serve as the Scientific Director of this Working Committee. Dr. Phelan is an Associate Professor at Children’s Wisconsin/Medical College of Wisconsin. She has served as Scientific Director of the Late Effects and Quality of Life Working Committee since March 2020. Saurabh Chhabra, MBBS, MD, who has been the Scientific Director of the Regimen-Related Toxicity and Supportive Care Working Committee since March 2017, will be leaving the Medical College of Wisconsin as he transitions to a new role at Mayo Clinic, Phoenix, AZ. We will plan to merge the proposals sent to both committees for consideration during this cycle. We plan to hold a combined MoReS Working Committee session during the 2023 Tandem Meetings in Orlando, FL. We hope to see many of you there!
We would like to thank all committee members for their dedication and commitment to participating in registry studies. Please do not hesitate to reach out to us with any questions.
Morbidity, Recovery, and Survivorship Working Committee Leadership:
Co-Chairs:
- David Buchbinder, MD (2023)
- Betty Hamilton, MD (2024)
- Hélène Schoemans, MD (2025)
- Edward Stadtmauer, MD (2023)
- Bipin Savani, MD (2024)
- Mohamed Sorror, MD, MSc (2025)
Scientific Director:
- Rachel Phelan, MD
Statistical Directors:
- Ruta Brazauskas, PhD
- Kwang Woo Ahn, PhD
Statistician:
- Joelle Strom, MS
CAC Representatives:
- Rebecca Higgins
- Brandon Nuechterlein
2023 Tandem Meetings

The Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR (Tandem Meetings) are the combined annual meetings of the ASTCT and CIBMTR. Administrators, clinicians, data manager / clinical research professionals, fellows-in-training, investigators, laboratory technicians, MD / PhDs, nurses, nurse practitioners, pharmacists, physician assistants, and other allied health professional attendees benefit from a full scientific program that addresses the most pressing issues in HCT and cellular therapy.
We look forward to hosting the 2023 Tandem Meetings in person at the World Center Marriott in Orlando, Florida. The 2023 Tandem Meetings will feature in-person and virtual programming, so everyone can experience the premier event in the evolving HCT and cellular therapy field.
Scientific Program
2023 Scientific Organizing Chairs, Stefanie Sarantopoulos, MD, PhD, and Sumithira Vasu, MBBS, along with the entire scientific organizing committee and session chairs have assembled a comprehensive program. An outline of topics is listed below. Throughout the 2023 Tandem Meetings, global experts in the HCT and cellular therapy field will present the latest developments during a combination of plenary and concurrent sessions as well as via oral abstracts, posters, specialized tracks, and more.
Scientific Program Topics
- Novel cellular therapy for heme malignancies: CAR-T & beyond
- Tumor microenvironment and relapse after immunotherapy
- Severe chronic GVHD: Lung, sclerotic, and brain manifestations
- Mechanisms in acute GVHD prevention: Applying what we now know
- Novel antibody-based conditioning regimens
- Lessons from the pandemic: Are we stronger?
- Mechanisms of relapse after cellular therapy and measures to address them
- Modulating host immune reconstitution: Vaccines and more
- Cell therapy for solid tumors
- Accelerated physiological aging after HCT
- How CAR-Ts drive the cell therapy treatment landscape for lymphoma patients
- Update on the global data for HCT
- Alternative grafts and donor sources: How to successfully transplant with a mismatched donor
- Treatment of refractory GVHD
- Statistical topics in design and analysis of cellular therapy and transplant studies
Plus: Mortimer M. Bortin lecture, E. Donnall Thomas lecture, late-breaking abstracts, CIBMTR Working Committee meetings, ASTCT spotlight sessions, and Meet-the-Professor sessions as well as industry-supported satellite symposia, product and innovation theaters, and an exhibit hall.
Tracks
Confirmed tracks include administrative director, advanced practice providers, BMT CTN coordinators, clinical research professionals / data management, new data manager onboarding, IT and informatics, nursing, pediatrics, and pharmacy.
Registration and Housing
Registration is now open with the option to register as either an in-person attendee or a virtual attendee. Once your registration is complete, you will receive information about how to book housing.
Support Opportunities and Additional Information
Please direct questions regarding support opportunities for the 2023 Tandem Meetings to the Tandem Meetings Conference Office: TandemMeetings@mcw.edu.
We look forward to seeing you in 2023!
Follow ASTCT and CIBMTR on social media, as well as the official hashtag, #Tandem23, for updates.
Bioinformatics Research Tools Benefit Users Around the World
By Michael Wright, PhD (Scientific Coordinator), and Pradeep Bashyal (Sr. Bioinformatics Software Architect)
The Bioinformatics Research group has created several web applications that allow the wider HLA matching and transplant community to carry out a range of tasks to improve their ability to find matches, improve the quality of donors found, and ultimately impact patient lives. Some of these tools were created years ago; for example, early versions of Haplostats have been available for more than ten years and have been added to and refined since then. Other tools have only recently been created, as the science supporting their use is based on recent discovery. For example, B-Leader is based on a paper published in 2020 (HLA-B leader and survivorship after HLA-mismatched unrelated donor transplantation by Effie Petersdorf, MD, and her group) and was made public in 2021. Because of the different time spans these tools have been available, they have different levels of public awareness and use. Some of the tools are newly created and still being tested before being made publicly available, whilst other tools are no longer used widely because science has progressed and better tools have replaced them.
Here we present the top five tools, as measured by the numbers of users throughout the last year.
| Tool | Users | URL | Description |
|---|---|---|---|
| Haplostats | 156,547 | haplostats.org | Search frequency information for haplotypes and haplotype pairs relative to specific HLA types found in the US population. |
| Frequencies | 2,427 | frequency.nmdp.org | Download high-resolution 6-locus haplotype frequencies derived from the entire DNA-typed NMDP registry for 21 detailed racial / ethnic categories and 5 broad categories. |
| Haplo Donor Selector | 1,000 | haplodonorselector.b12x.org | Predict the probability of disease-free survival from attributes of a HCT donor-recipient pair (Fuchs E et al., 2021). |
| B-leader | 841 | bleader.nmdp.org | Sort single HLA-B mismatches based on the HLA-B leader peptide for unrelated and/or haploidentical donors for HCT. (Sajulga et al., 2021). |
| Search Prognosis | 234 | search-prognosis.b12x.org | Conduct a quick search prognosis at the onset of an adult unrelated donor search. This tool provides a simple scoring system based on a patient's race / ethnicity and genotype frequency. It could be a useful tool for transplant physicians to understand the difficulty of identifying a fully matched or suitably mismatched unrelated donor. |
When we look more closely at how the most frequently used tool, Haplostats, was used over the last year, the numbers fluctuate as different groups use it intensely for various projects. The most uses in a single day was just under 8,000 (equating to one use every 11 seconds), which indicates that researchers are so keen to get the information that they have written scripts to automatically process many HLA typings in as short a time as possible.
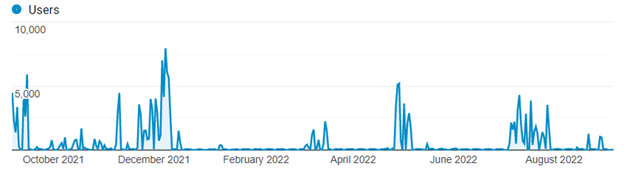
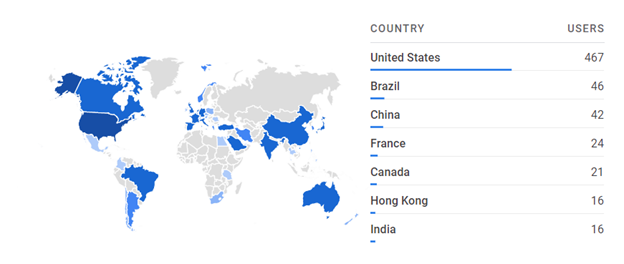
When evaluating user location, we can see that for Haplostats most users are from Canada. For other tools, it is interesting to look at the city with the most uses and relate that to medical centers. For example, we can tell that someone was investigating T-cell-epitope groups using one of our newer tools.
It is also important to remember that matching is an international process, so users are scattered across the globe. A perfect example is displayed below, showcasing the users for the B-leader tool:
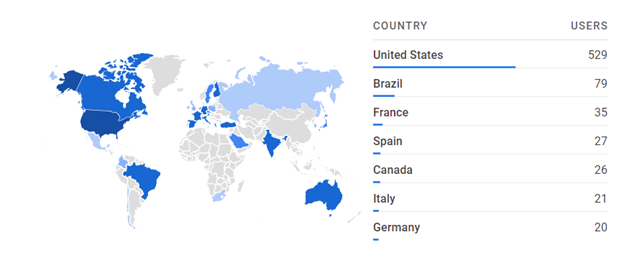
In both cases, it is clear that there are a number of countries where the tools are used infrequently, but each use represents someone wanting to find the best match to help save someone's life.
The official website for the Bioinformatics group can be found at: https://bioinformatics.bethematchclinical.org/ (the bioinformatics section on bethematchclinical.org). This site currently does not host individual tools but provides HLA resources as well as links to the tools and data standards (for example HML). Last year there were 46,277 users, the majority of which were from the US, with the next largest group of users from Germany.
The team is in the process of updating the website. Soon you will be able to find information and links to all the tools and resources built and supported by the team, including upcoming applications.
Health Services Research Program
By Jaime Preussler, Christa Meyer, Rafeek Yusuf
The Health Services Research Program within CIBMTR NMDP studies the access, utilization, value (quality and cost), delivery, organization, financing, and outcomes of HCT and other cellular therapies to generate new knowledge and improve understanding of the structures, processes, and impacts of these therapies on individuals with hematologic cancers and other blood disorders.
The Health Services Research Program draws on a multitude of approaches including, but not limited to, retrospective and prospective research, administrative claims data analysis, survey design and administration, mixed (qualitative and quantitative) methods, dataset linkage and integration, and geographic information systems mapping.
The research program is now a standalone program within NMDP, with a dedicated team of researchers possessing extensive experience in health services research.
Select studies include:
- Research using CMS administrative claims data to learn more about physician referral and treatment patterns
- Evaluation of racial / ethnic differences in utilization of allogeneic HCT in AML in California using a novel linked dataset
- Focus groups with social workers and interviews with patients, caregivers, and physicians / advanced practice professionals to learn more about caregiver requirements and perspectives on those requirements
Findings from these and other ongoing studies will help inform the development of future programs and interventions to help support patients, caregivers, health professionals, and transplant centers.
To learn more about this research program, visit the Health Services Research Program webpage or email Rafeek Yusuf.
CIDR: Beyond the Finish Line
As we enter the fifth and final year of the CIDR award, we would like to take the time to reflect upon the immense work and dedication we've witnessed over the past months. As you may have heard, we recently submitted the U24 HCT infrastructure grant that will support the continuity of CIDR initiatives beyond the original grant's lifecycle. In response to the CIBMTR's achievements, the NIH has recognized our efforts through continued support to collect data for HCT and adoptive cell therapy, which includes cellular therapies like CAR-T.
This collaborative effort has been built by past and present leaders of the CIBMTR. Our institution is uniquely positioned to serve the community of patients, centers, researchers, clinicians, and many other stakeholders. We believe the CIBMTR has influenced the improvement of access to and outcomes of patients receiving HCT over the years, and we will continue to lead efforts on the forefront, such as expanding our efforts to include capturing data on adoptive cellular therapies. This belief drives our investment toward ensuring that CIBMTR is successful in pursuing its mission.
The organization, attention to detail, and drive to succeed we've witnessed in the preparation of the U24 submission confirms that we are surrounded by friends that share this belief. We are excited and curious to see how the CIBMTR will continue to achieve its mission.
CIDR Year 5 Goals
During the last year of the CIDR grant, the focus is to ensure the continuation and advancement of many initiatives. This grant year, we plan to:
- Launch data collection tools focused on solid tumors
- Operationalize the data embargo tool to allow centers to temporarily embargo pre-specified clinical trial data in the registry to incentivize centers to report pre-commercial data to the CIBMTR and use its infrastructure for long-term follow-up protocols
- Pilot data collection at the time of leukapheresis to assess dropout from collection to infusion of cells
- Continue enrollment of cellular therapy recipients to the PRO protocol
- Formalize the cellular therapy audit metrics to align with CIBMTR standards
- Establish mechanisms to support consortia development
We plan to continue previous initiatives that aim to improve data collection, quality, and accessibility to the resource for the benefit of our communities.
2022 AcCELLerate Forum
The second annual virtual AcCELLerate Forum: Creating a Sustainable Ecosystem of Cell and Gene Therapy, is scheduled for November 17-18, 2022. ASTCT, CIBMTR, and NMDP/Be The Match have collaborated to host this two-day virtual workshop offering educational and advocacy opportunities for providers, payers, government agencies, and industry members in the cell and gene therapy field. Please visit the AcCELLerate Forum website for additional information and to register for this exciting event.
Stem Cell Therapeutic Outcomes Database
By Carol Doleysh
The SCTOD is part of the US Health Resources and Services Administration (HRSA)-funded C.W. Bill Young Cell Transplantation Program that collects data on all allogeneic HCTs performed in the US and on transplants done elsewhere using cellular products that originated in the US.
Impact of the COVID-19 Pandemic on the Center-Specific Survival Analysis
One of the important topics at the 2021 Center Outcomes Forum was handling the impact of the COVID-19 pandemic in the Center-Specific Survival Analysis. Following recommendations from that meeting, several exploratory analyses to assess the influence on outcomes of pandemic-related factors (pandemic time period, geographic COVID-19 incidence rate, geographic mortality rate) for HCT recipients in 2019 and 2020. Based on the results of those analyses, recipients who developed COVID-19 infection during their first year of follow-up were not censored in the model for the 2022 analysis, which included HCTs during 2018-2020. Results of the 2022 analysis are scheduled for public release in December 2022.
HRSA Renews SCTOD Contract
The CIBMTR successfully competed for, and was awarded, a renewal of the SCTOD contract with HRSA. This contract was first awarded to CIBMTR in 2006. The outcomes registry of the CIBMTR currently contains information for more than 600,000 transplant recipients as well as essential data to continually evaluate operations of the national transplant program, known as the C.W. Bill Young Cell Transplantation Program.
PRO Data Now Available in DBtC
On Friday, September 23, 2022, the DBtC platform began sharing PRO data collected under CIBMTR's PRO Protocol. DBtC includes patient scores on 8 individual PROMIS measures, and visualizations of average scores by time point (baseline, day 100, day 180, 1 year, 2 years). Centers can view data about their patients who are participating in the PRO Protocol and previously agreed to share data with their medical providers. We are excited to share this new data resource with the HCT and cellular therapy community. Please email the PRO Protocol mailbox at PRO-Surveys@nmdp.org with any questions about the CIBMTR's PRO collection.
To avoid duplicating PRO efforts and to make sure we understand unique barriers, we ask centers to agree to allow the CIBMTR to approach their patients for this protocol. This is open to any US center treating adult patients.
Please let us know if we can include your patients! Complete this brief survey to request more information about the protocol and express your interest in including your patients. You can also reach out to the protocol mailbox, PRO-Surveys@nmdp.org, with any questions.
MEASURE Now Enrolling

NMDP/Be The Match and the CIBMTR are excited to announce the opening of accrual and the first enrollments to the Molecular Evaluation of AML Patients After Stem Cell Transplant to Understand Relapse Events (MEASURE) protocol NCT05224661! This new study is managed by the RCI BMT, in partnership with the NHLBI
While transplant is a curative therapy for many patients with AML, the risk of relapse remains high after transplant. The MEASURE study's goal is to determine the optimal post-transplant monitoring strategy for AML patients to predict and better treat relapse. This will be accomplished by creating a coordinated national framework to prospectively collect samples, building on a previous study, which demonstrated that the presence of MRD prior to transplant was associated with increased rates of relapse.
Over the next 4 years, the study will enroll 1,000 participants receiving allogeneic HCT to treat AML and follow them for 3 years post-transplant, with the goal of study results translating into the following lifesaving practices:
- Harmonize MRD testing across US transplant centers
- Develop personalized strategies for post-transplant monitoring of AML patients to detect relapse sooner
- Assist physicians in making clinical decisions, like the best conditioning regimens or post-transplant therapy for patients based on AML type
Results from the Pre-MEASURE study were presented at ASCO, made the ASH Clinical News top-5 most-read list for July 2022, and are currently under review for publication in The New England Journal of Medicine.
Additionally, the MEASURE study is piloting the use of a custom-built LabVantage Laboratory Inventory Management System (LIMS) module to manage the shipment of samples between study sites, the NMDP/Be The Match Repository, and study partner labs. LabVantage LIMS has been used by the NMDP/Be The Match Repository to manage the CIBMTR and BMT CTN research sample inventory since 2015.
The study protocol includes as many as 12 sample collection time points for each participant. With a projected enrollment of 1,000 subjects, sample management will be critical to the success of this study. RCI BMT immunobiology, data management, CIBMTR Information Technology, and NMDP/Be The Match IT have put in tremendous effort collaborating over the last year to make this platform a reality for our RCI BMT prospective clinical trials, for both this study and future protocols.
Share Your Research
By Jennifer Motl
This new plain-language summary of CIBMTR research may help your patients:
 |
Younger BMT donors sometimes better than older donors Blood and marrow transplant helps people with myelodysplastic syndromes. Read more: accessible or 1-page version
|
Find more summaries on the Study Summaries for Patients webpage.
Publicly Available Datasets
NEW: View a summary of the Publicly Available Datasets and the data dictionary containing the most commonly used variables.
In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, the CIBMTR makes the final datasets from published studies publicly available on the CIBMTR Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
While providing these data, the CIBMTR is committed to safeguarding the privacy of participants and protecting confidential and proprietary data. Upon accessing the datasets page on the CIBMTR public website, the viewer is notified that the dataset was collected by the CIBMTR, and the CIBMTR's supporters are listed. The webpage also clearly notes the terms and conditions of dataset usage.
NEW datasets are now available online.
Our Supporters
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 and U24HL157560 from NHLBI and NCI; U24CA233032 from the NCI; OT3HL147741 from the NHLBI; HHSH250201700005C, HHSH250201700006C, and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research.
Additional federal support is provided by P01CA111412, R01CA100019, R01CA152108, R01CA218285, R01CA231141, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01HL155741, R01HL131731, UM1CA121947, U01AI069197, U01AI126612, UG1HL06924.
Support is also provided by Be The Match Foundation; Boston Children's Hospital; Dana Farber; St. Baldrick's Foundation; PBMTF; Stanford University; Medical College of Wisconsin; National Marrow Donor Program; and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon, a Sanofi Company; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Sanofi; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
The views expressed in this newsletter do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA), or any other agency of the US Government.
The CIBMTR® is a research collaboration between the National Marrow Donor Program (NMDP)/Be The Match and the Medical College of Wisconsin.
