August 2024 Newsletter

Volume 30, Issue 3
Table of Contents:
-
Working Committee Training and Leadership Program: Meet 2 More Participants
-
Data Transformation Initiative: Cytogenetic and Medication Data Updates
-
HLA_SAVE: An Essential Resource for Transplant-Associated Immunogenetic Research
-
Health Services Research: Caregiver Requirements for Allogeneic HCT
Perspectives:
Vedolizumab for GI GVHD Prevention: Balancing Efficacy, Cost, and Clinical Implementation
By Michael Verneris, MD

Gastrointestinal (GI) GVHD is a severe and potentially life-threatening complication following allogeneic HCT. Patients suffering from GI GVHD experience significant morbidity, which profoundly impacts their quality of life. The symptoms of GI GVHD, including severe abdominal pain, diarrhea, nausea, vomiting, and gastrointestinal bleeding, can be debilitating. These symptoms not only cause immense physical suffering but also lead to psychological distress and reduced overall well-being.
The burden of GI GVHD extends beyond patient suffering to substantial medical resource utilization. Hospitalizations for severe GI GVHD are often prolonged, requiring intensive medical management, including intravenous fluids, nutritional support, and immunosuppressive therapies. The need for frequent monitoring and the prevention and / or management of complications, such as infection, further escalates healthcare costs. Moreover, severe GI GVHD increases the risk of treatment-related mortality and adversely affects transplant outcomes, leading to increased healthcare utilization and economic burden.
The Promise of Vedolizumab in Preventing GI GVHD
Recent advances in understanding the pathophysiology of GI GVHD have paved the way for targeted therapies aimed at preventing this debilitating complication. One such promising approach is the use of vedolizumab, a monoclonal antibody that blocks integrin α4β7. This adhesion receptor plays a crucial role in the homing of T cells to the gut, a key step in the development of GI GVHD.
A recent Phase III study published in Nature Medicine by Chen et. al. investigated the efficacy of vedolizumab in preventing GI GVHD. In this randomized, double-blind, placebo-controlled trial, patients receiving a calcineurin inhibitor and either methotrexate or mycophenolate mofetil were administered vedolizumab (or placebo) at specific days -1, +13, +41, +69, +97, +125, and +153 before and after transplant.
Excitingly, the study demonstrated that vedolizumab was well-tolerated and met its primary endpoint of reducing lower GI GVHD-free survival at day 180, with a hazard ratio of 0.45. Importantly, vedolizumab did not adversely impact other key transplant outcomes, including relapse. While decreasing severe lower GI GVHD, the cumulative incidence of treatment-related mortality, chronic GVHD, and overall survival was not improved. These findings are significant, suggesting that vedolizumab can effectively reduce the incidence of GI GVHD without compromising the overall success of the transplant.
Translating Clinical Trial Results to Practice
While the results of the vedolizumab trial are indeed exciting, translating these findings into clinical practice presents several unanticipated challenges to me. As a pediatrician, reading this study made me recognize the complexity of adopting new therapies based on Phase III clinical trial data, especially when the study population differs significantly from my patient cohort.
One of the primary considerations is the demographic differences between the trial participants and my patients. The study enrolled nearly all adults and with the goal of homogeneity, it did not include patients receiving umbilical cord blood or haploidentical transplants with post-transplant cyclophosphamide. Additionally, while the subgroup analysis did not show differences in outcomes based on HLA match, conditioning intensity, use of ATG, or stem cell source, it is noteworthy that most patients received PBSCs and underwent RIC, whereas many of my pediatric patients receive MAC and a significant proportion have non-malignant indications for transplant.
Given these differences, it is challenging to evaluate whether these findings are applicable to my patient population. Although vedolizumab showed a clear benefit in preventing GI GVHD in the trial, the dosing schedule and its efficacy in pediatric patients, as well as those with different transplant protocols, need further investigation.
Dosing Considerations and Cost Implications
While the schedule appears tolerable, it raises questions about whether all the doses are necessary or if the regimen could be further optimized. In practice, most of my patients who develop GI GVHD seem to do so relatively early in their transplant course, suggesting that a more condensed dosing schedule might be acceptable. However, this hypothesis requires validation through further studies.
Cost is another significant consideration. The financial burden of vedolizumab is substantial, with costs to incorporate this into prophylactic regimens potentially reaching 5 to 6 digits. Further, I question whether I am obligated to discuss such a change with hospital administration and pharmacy groups to address budgeting and resource allocation. Despite the high cost, the potential to reduce severe GI GVHD and improve patient outcomes could justify the expense, particularly when considering the long-term healthcare savings from preventing severe GVHD-related complications.
Evaluating the Broader Impact
While the Phase III study offers promising results, somewhat surprisingly to me, I have come to realize that one study alone does not provide a comprehensive solution. The trial's findings need to be corroborated by further research, including studies involving diverse patient populations and different transplant protocols. As a physician, I feel that I must weigh the benefits demonstrated in the trial against the uncertainties presented above, and incorporating vedolizumab into clinical practice should be approached with caution.
Conclusion
The prevention of GI GVHD remains a critical goal in improving the outcomes of allogeneic HCT. By targeting the integrin α4β7, vedolizumab offers a promising approach to reducing the incidence of this debilitating condition. This Phase III study provides compelling evidence for its efficacy and safety, marking a significant advancement in the field. However, translating these results into routine clinical practice involves several considerations, including patient population differences, dosing schedules, cost implications, and the need for further validation.
Ultimately, this exercise in considering how to incorporate vedolizumab into practice represents a journey from clinical trial results to practical application and shows that the implementation of “progress” is complex and multifaceted. It underscores the importance of ongoing research, collaboration, and thoughtful implementation to achieve the best possible outcomes for our patients. While one Phase III study is a significant step forward, it is not the final solution and continuous efforts are needed to refine and optimize prevention strategies for GI GVHD.
Non-Malignant Diseases Working Committee
Committee Leadership
Co-Chairs:
- Ashish Gupta, University of Minnesota Blood and Marrow Transplant Program – Pediatrics, Minneapolis, MN
- Carmem Bonfim, Hospital Pequeno Príncipe, Curitiba, PR
- Kasiani Myers, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
Scientific Director:
- Larisa Broglie, CIBMTR MCW
Statistical Director:
- Soyoung Kim, CIBMTR MCW
Statistician:
- Yongzi Yu, CIBMTR MCW
The Non-Malignant Diseases Working Committee conducts clinical research on early and late outcomes following allogeneic HCT for these diseases. Many of the diseases are rare to ultra-rare, and the patient population is small; therefore, the best avenue to collect maximum information about HCT outcomes for these diseases is to promote multi-institutional collaborative studies. This working committee collaborates with EBMT, Eurocord, and individual highly specialized centers to report transplant-related topics, advance the field of knowledge, and guide practice in this area. The disorders covered by the committee are broadly classified under the following categories: Marrow failure (acquired and inherited), hemoglobinopathies, metabolic disorders, immune deficiency / dysregulation disorders, and autoimmune diseases. The uniqueness of this working committee is that we manage a wide variety of diseases. However, these diseases also have much in common. For example, the majority of patients with non-malignant diseases who undergo transplant-based therapies are young (including infants), so the toxicities may be different from those seen in adults, and the potential to positively affect their lives is magnified.
The Non-Malignant Diseases Working Committee leadership includes experts in the field who bring their unique perspectives to help develop high-impact studies. Carmem Bonfim, MD, PhD, joined the committee’s leadership this year from the Federal University of Paraná in Curitiba, Brazil, bringing her expertise in transplant for non-malignant diseases, including Fanconi anemia and aplastic anemia. Ashish Gupta, MBBS, MPH, also joined the committee’s leadership this year from the University of Minnesota, bringing his expertise in transplant and gene therapies for hemoglobinopathies and inherited metabolic diseases. Kasiani Myers, MD, enters her second year as a co-chair, bringing her expertise in inherited bone marrow failure syndromes and experience at Cincinnati Children’s to the committee. The statistical team includes Yongzi Yu and Dr. Soyoung Kim, PhD, and the committee’s scientific director is Larisa Broglie, MD, MS. The committee also participates in CIBMTR’s Working Committee Training and Leadership Program with Brian Ball, MD, from City of Hope entering his second year participating in this program.
In the last 4 years (2020-2024), the committee produced 6 peer-reviewed manuscripts. Currently, multiple studies are ongoing. Three studies have manuscripts under revision, with plans to submit for publication soon:
- A study on long-term outcomes of 123 patients who received autologous HCT at 10 centers in the US and Canada for systemic sclerosis (scleroderma) with follow-up of up to 20 years
- A study of trends in outcomes of patients with Fanconi anemia, assessing factors that have impacted those trends
- A study of outcomes of transplant for patients with erythropoietic porphyria, a collaborative study with EBMT
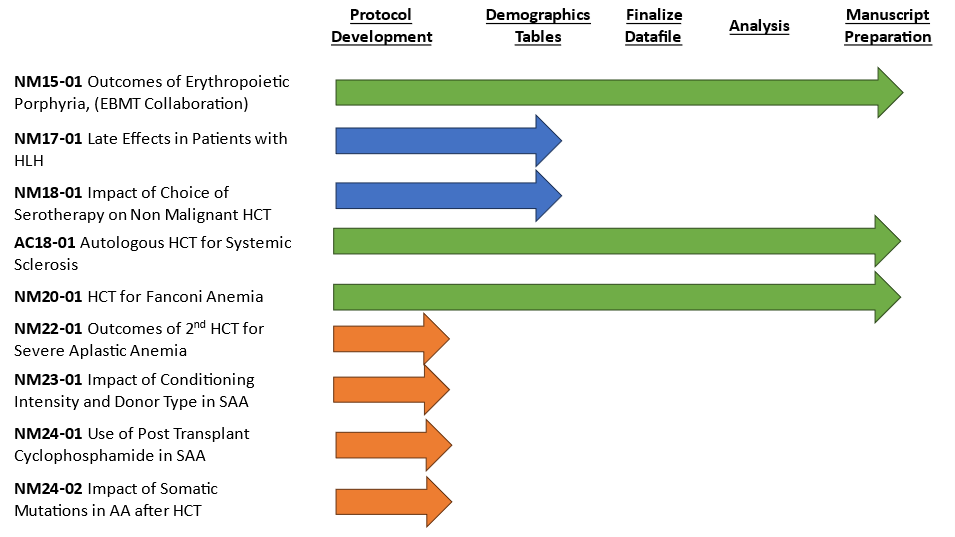
Two other studies are in the process of finalizing the protocol and developing demographic tables:
- An evaluation of serotherapy approaches during transplantation for non-malignant diseases
- Late effects of patients with hemophagocytic lymphohistiocytosis (HLH)
Four studies focusing on patients with aplastic anemia are currently in protocol development and awaiting incorporation of aplastic anemia data as part of CIBMTR’s recent data transformation efforts. The protocols are planned to be finalized, demographics tables developed, and datafiles prepared starting in the fall of 2024.
Committee membership draws investigators from diverse backgrounds, each with experience in transplantation for benign, often rare disorders. The uniquely different disorders and the common transplant goals provide a great opportunity for synergy in scientific interactions and for members to bring forth new ideas. A full list of the studies undertaken by this committee, including recent publications, is provided on the Non-Malignant Diseases Working Committee studies webpage. Each year, 1-2 new proposals are selected during the Tandem Meetings to proceed with protocol development. The selection process includes input from the working committee members who score and prioritize studies based on interest and impact factors. We encourage active participation via the submission of new proposals and provision of input on studies at the protocol, analysis, and manuscript phases of the study. The committee’s success is highly dependent upon the scientific community. We also encourage junior investigators to provide input. To learn more about our committee or to discuss ideas and new projects, please contact one of the committee’s co-chairs or the scientific director.
Donor and Recipient Health Services Working Committee
Committee Leadership
Co-Chairs:
- Fotios Michelis, Princess Margaret Cancer Center, Toronto, OhioHealth, Columbus, OH
- Hemalatha Rangarajan, Nationwide Children's Hospital, Columbus, OH
- Leslie Lehmann, Dana-Farber Cancer Institute – Peds, Boston, MA
- Minocher Battiwalla, Sarah Cannon Transplant and Cellular Therapy Program at TriStar Centennial Medical Center, Nashville, TN
Scientific Director:
- Heather Stefanski, CIBMTR NMDP
Statistical Director:
- Brent Logan, CIBMTR MCW
Statistician:
- Gabrielle Schmidt, CIBMTR NMDP
The Donor and Recipient Health Services Working Committee was formed this year by merging two committees: The Donor Health and Safety Working Committee and the Health Services and International Studies Working Committee. CIBMTR merged these two committees to encourage new collaborations and novel research ideas. The Donor and Recipient Health Services Working Committee also works with the NMDP-supported Health Services Research Program, part of the NMDP Implementation Science team.
Our committee focuses on social determinants of health-related factors affecting transplant and cellular therapy and studies how these factors influence treatment access, utilization, and outcomes. At times, this includes merging databases outside of CIBMTR with CIBMTR’s database to truly understand all factors that can affect transplant outcomes and ensure both patient and donor safety. Examples of other databases are CMS (both Medicare and Medicaid), publicly available datasets (e.g., American Community Survey data and social vulnerability index), and state cancer registry data.
Our committee recently published 3 manuscripts:
- “The association of ABO mismatch with the outcomes of allogeneic hematopoietic cell transplantation (HCT) for acute leukemia” was published in the American Journal of Hematology. The study cohort had almost 5,000 allogeneic HCT recipients with AML and ALL. Major ABO mismatch was associated with worse overall survival, inferior platelet engraftment, and higher graft failure rate.
- “Racial and socioeconomic disparities in long-term outcomes in ≥1 year allogeneic hematopoietic cell transplantation survivors: A CIBMTR analysis” was published in the Transplantation and Cellular Therapy journal. This study revealed no association between race / ethnicity or poverty level in relation to overall survival, progression-free survival, relapse, and non-related mortality.
- “Trends in volumes and survival after hematopoietic cell transplantation in racial/ethnic minorities” was published in Blood Advances. This study examined the volume and rates of change of allogeneic and autologous HCT in 79,904 autologous and 65,662 allogeneic HCTs, and the trends in overall survival in 4 racial / ethnic groups: Non-Hispanic Whites, non-Hispanic African Americans, and Hispanics across 5 cohorts from 2009 to 2018. The number of autologous HCT and allogeneic HCT grew faster in non-Hispanic African Americans and Hispanics than in non-Hispanic Whites. Survival after autologous HCT and allogeneic HCT improved over time for all racial / ethnic groups, though African Americans had worse outcomes.
We have two studies under review:
- Relationship of race / ethnicity and survival after single and double umbilical cord blood transplantation
- International collaborative study to compare the prognosis for acute leukemia patients transplanted with intensified myeloablative regimens
Several other studies are in various stages of protocol development and analysis.
We are also lucky to have Megan Herr, PhD, on the committee as a participant in the Working Committee Training Leadership Program. Dr. Herr has been a huge asset to the committee. She is involved in the monthly meetings, updating the CIMBTR forms as they relate to social determinants of health questions. Dr. Herr will also be working with co-investigators on the recently accepted 2024 study proposal, “Outcomes for Medicaid beneficiaries following hematopoietic cell transplantation and the potential impact of variable Medicaid eligibility criteria.”
The Donor and Recipient Health Services Working Committee strives to conduct studies that help address healthcare inequities, understand barriers, and find ways to reduce the existing gaps in access to transplant and cellular therapy for all patients.
Save the Date for the 2025 Tandem Meetings!

By Alicia Halfmann and Maira Brey
The 2025 Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR will be held February 12-15, 2025, with pre-conference events on Tuesday, February 11, and post-conference events on Sunday, February 16, in Honolulu, Hawaii.
Current information regarding the 2025 Tandem Meetings can be found at TandemMeetings.com, including details about scientific sessions, exhibit and support information, and registration, which is set to launch in fall 2024! Please send any questions regarding the Tandem Meetings to TandemMeetings@mcw.edu.
Follow #Tandem25 for the latest updates!
Working Committee Training and Leadership Program: Meet 2 More Participants
The Working Committee Training and Leadership Program was first announced during the 2023 Tandem Meetings. We had an overwhelming response, receiving applications from 77 Early Career Investigators representing more than 65 distinct transplant and cellular therapy centers in 10 countries. The eight participants selected all share a high level of dedication, achievement, and enthusiasm for clinical research. We would like to warmly welcome them to the program and are excited to be a part of their career development. Join us as we continue to highlight the participants in this edition of the newsletter.
Megan Herr, PhD: Donor and Recipient Health Services Working Committee
 I am an associate member in transplant and cellular therapy in the Department of Medicine at Roswell Park Comprehensive Cancer Center. My research primarily focuses on transplant and cellular therapy morbidity, but as a clinical epidemiologist, I also oversee the data management team responsible for CIBMTR forms reporting. Thus, I have unique insight into the CIBMTR case report forms and how the data are abstracted from primary data sources such as the electronic medical record and interpreted from clinical notes. My goal in being part of CIBMTR’s Working Committee Training and Leadership program was to learn how the organization prioritizes its workflow and the study process – from proposal selection to manuscript preparation. This opportunity has exceeded my expectations in terms of networking opportunities and valuable insights from experts in the field.
I am an associate member in transplant and cellular therapy in the Department of Medicine at Roswell Park Comprehensive Cancer Center. My research primarily focuses on transplant and cellular therapy morbidity, but as a clinical epidemiologist, I also oversee the data management team responsible for CIBMTR forms reporting. Thus, I have unique insight into the CIBMTR case report forms and how the data are abstracted from primary data sources such as the electronic medical record and interpreted from clinical notes. My goal in being part of CIBMTR’s Working Committee Training and Leadership program was to learn how the organization prioritizes its workflow and the study process – from proposal selection to manuscript preparation. This opportunity has exceeded my expectations in terms of networking opportunities and valuable insights from experts in the field.
Najla El Jurdi, MD: GVHD Working Committee
 I am an adult transplant physician who recently joined the NIH / NCI as an associate research physician after 5 years at the University of Minnesota. My clinical and research focus is allogeneic HCT and cellular therapies for myeloid malignancies, with particular interest in GVHD and late effects. CIBMTR’s Working Committee Training and Leadership Program has proven to be a tremendously valuable career experience. I have the unique opportunity to meet and closely work with experts and role models in the field, learning approaches to advance quality clinical research. As a program member, I have access to in-depth knowledge of CIBMTR’s rigorous multidisciplinary work that is necessary to create a robust and inclusive database, followed by careful proposal selection and optimization, statistical insights and data analysis, and manuscript development by the working committee. Participating in this program has also increased my awareness of the richness of the biorepository that can be leveraged for future research. I look forward to continuing to learn from our enthusiastic and productive working committee scientific directors and co-chairs.
I am an adult transplant physician who recently joined the NIH / NCI as an associate research physician after 5 years at the University of Minnesota. My clinical and research focus is allogeneic HCT and cellular therapies for myeloid malignancies, with particular interest in GVHD and late effects. CIBMTR’s Working Committee Training and Leadership Program has proven to be a tremendously valuable career experience. I have the unique opportunity to meet and closely work with experts and role models in the field, learning approaches to advance quality clinical research. As a program member, I have access to in-depth knowledge of CIBMTR’s rigorous multidisciplinary work that is necessary to create a robust and inclusive database, followed by careful proposal selection and optimization, statistical insights and data analysis, and manuscript development by the working committee. Participating in this program has also increased my awareness of the richness of the biorepository that can be leveraged for future research. I look forward to continuing to learn from our enthusiastic and productive working committee scientific directors and co-chairs.
“Dr. El-Jurdi is a great addition to the GVHD Working Committee leadership team. She is always eager to help with any committee work, no matter how mundane, and provides important comments when we are reviewing the ongoing studies." ~ Stephanie Lee, MD, MPH, Scientific Director of the GVHD Working Committee
Data Transformation Initiative: Cytogenetic and Medication Data Updates
DTI continues to expand the amount of data that can be exchanged electronically, to reduce the burden of manual data submission for transplant centers, and to provide the data needed for CIBMTR research and reporting. CIBMTR consistently onboards and engages transplant centers and partners with DTI, and interest continues to grow. More than 28 centers and partners are currently onboarded with DTI.
In the past year, the DTI team introduced the ability to capture the International System for Human Cytogenomic Nomenclature (ISCN) string for conventional cytogenetic results. This functionality reduces the burden of reviewing and interpreting the results and allows transcription of the ISCN string. Once the ISCN string is collected within FormsNet3, the systems interpret and parse the cytogenetic abnormalities, work that was previously done by transplant center staff. The functionality is captured on the Pre-TED Disease Classification (Form 2402) and 8 disease classification forms. As forms that collect cytogenetic information are revised, this new functionality will be added.
In May 2024, the DTI team released new functionality allowing participating centers to electronically submit medication-related data directly from Epic using CIBMTR’s SMART on FHIR (Fast Healthcare Interoperability Resources) app and HL7 FHIR. These data undergo automated processing and selection into CIBMTR’s outcomes database via FormsNet. With this functionality, data managers are relieved from the manual data entry burden for medications within the following sections of the Pre-TED (Form 2400): Preparative regimen, GVHD prophylaxis, peri-transplant period, and prior drug exposure. The medication-related data submission ability extends previously existing functionality for automated submission of CIBMTR patient registration, lab panels, and HLA data. The DTI team continuously upgrades and improves systems that support greater data automation, to find efficiencies in data collection and downstream data use for research purposes.
As the year progresses, CIBMTR will continue to enhance automated data capture, with the goal of reducing burden and increasing data submission efficiency for participating centers and their data managers.
OPTIMIZE Update Off to a Quick Start!
NMDP is excited to announce the quick start of the new OPTIMIZE (NCT06001385) study! Since enrolling the first patient in January 2024, the study has enrolled more than 50 patients in the study – exceeding expectations by 86%. The OPTIMIZE study is a Phase II trial evaluating reduced dose post-transplant cyclophosphamide (RD-PTCy) in adult recipients of a PBSC product from a MMUD.
PTCy, which is commonly used as a GVHD prophylaxis agent, can cause potentially life-threatening complications, including organ toxicities and severe infections. By lowering the dose of PTCy, OPTIMIZE hypothesizes that the rate and severity of these PTCy-related complications will decrease while maintaining comparable protection against GVHD.
The study is now open at 15 participating centers across the US with start-up in progress at 18 additional centers. OPTIMIZE plans to complete enrollment of all 187 planned subjects by January 2026.
HLA_SAVE: An Essential Resource for Transplant-Associated Immunogenetic Research
By Valerie Stewart, PhD
HLA_SAVE is a cumulative data extract capturing demographic and immunogenetic data from donors and recipients of allogeneic HCT reported to CIBMTR. A unique and invaluable resource, HLA_SAVE offers the ability to study the impact of immunogenetics alone or as a variable in HCT outcomes. Having been incorporated into more than 200 published studies, its data have been foundational in establishing modern match guidelines. Delivered quarterly by the Immunobiology Research Program, with support from Business Intelligence, Bioinformatics, and Biostatistics, HLA_SAVE continues to amass a wealth of transplant-associated data, including improvements in HLA quality resulting from retrospective typing data from recent transplants.
Representing the extent of immunogenetic similarity between donor and recipient, the research match grade variable (MGV) is one of the most critical elements of the HLA_SAVE dataset. Specifically, MGV reflects the number of allele matches at a specified number of HLA genes (loci); values of n/6, n/8, n/10, and n/12 are reported to indicate donor-recipient match at as many as six loci: HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Assignment of the MGV requires determination of match grade at individual HLA loci. However, the historical method of match grade calculation used in dataset preparation was complicated, inefficient, and outdated. Developed by the NMDP Bioinformatics team, py-ard offers an opportunity to modernize HLA_SAVE production by providing a method to transform HLA data from historical sources and reduce it to the antigen recognition domain for MGV assignment. Offering a modern, stable codebase for the future, py-ard accesses the IPD/IMGT-HLA database to assign values reflective of the most recent version of the HLA allele catalog.
Deployed in the March release of HLA_SAVE, py-ard implementation required extensive inter-team collaboration. Due to the substantive nature of the change, the Bioinformatics team deferred release to December to ensure robust quality assurance and unprecedented data validation efforts. The team confirmed report alignment between releases through investigation, reconciliation, and documentation of every MGV change. Towards our commitment to increased transparency and documentation, all changes and the results of our quality assurance assessment are reported in the March Release Notes in addition to reference documentation surrounding our algorithm for research MG assignment. We believe that the new approach reflects the current allelic inventory and more accurately assigns matches of clinical relevance.
With this release, we are proud to further extend the research value of our dataset through the inclusion of additional variables; in response to community feedback, HLA_SAVE now includes data surrounding disease and B-leader, an immunogenetic factor whose match status is associated with transplant risk. As always, we welcome feedback from the community, including suggestions for future enhancements, which can be directed to Valerie Stewart (vstewar2@nmdp.org).

Race, Ethnicity, and Ancestry
By Abeer Madbouly, PhD
Race, ethnicity, and ancestry are often misinterpreted and / or used interchangeably. There is a lack of consensus in the scientific literature on the definition of these terms and insufficient guidelines on the proper classification, collection, and application of this data in the scientific community. However, defining groups for human populations is crucial for multiple healthcare applications and clinical research. At NMDP / CIBMTR, our work to find a matched / mismatched donor for a patient seeking a stem cell transplant or cellular therapy is impacted by population classification, which relies mostly on self-reported data we receive from registry members at the time they join the registry.
Published in March 2024, an article authored by Abeer Madbouly, PhD, and Yung-Tsi Bolon, PhD, 1 describes aspects of race, ethnicity, and ancestry information that impact the stem cell or solid organ transplantation field with a focus on HLA data collected from donors and recipients by donor registries or transplant centers. The article lists the definitions and differences between the often-confused terms “race," "ethnicity," and "ancestry" and describes multiple applications of these data collected from registry members or transplant recipients. NMDP efforts to improve the collection and mapping of these data over the years are also narrated, including the evolution of population groupings and multiple social and political influencers that caused changes in these groups, and how they impacted the quality of data collected.
Importantly, detailed examples of direct applications to registry operations and government reporting are illustrated, including donor-recipient matching, statistical imputation, haplotype frequency estimation, and match rate projections. The new ancestry categories, deployed in operations in the summer of 2020 for donor recruitment, are reported in the article together with some ongoing research conducted by the Bioinformatics team to improve the processes and applications linked to these important data. Since publication, this article has been viewed or downloaded more than 2,000 times.
1 Madbouly A, Bolon YT. Race, ethnicity, ancestry, and aspects that impact HLA data and matching for transplant. Frontiers in Genetics. 2024 Mar 15; 15:1375352. doi: 10.3389/fgene.2024.1375352. PMC10978785.
Health Services Research: Caregiver Requirements for Allogeneic HCT
The NMDP-supported Health Services Research Program, in partnership with CIBMTR, explores how socioeconomic factors, health behaviors, financial systems, and healthcare processes affect access to and outcomes of cellular therapies. The Health Services Research Program conducts research through both qualitative and quantitative methods.
A recently completed study involved conducting focus groups and interviews to better understand caregiver requirements for allogeneic HCT. This research informed a proposal to convene stakeholders in a series of workshops to create a patient-centered research agenda to redefine the caregiver paradigm for safe post-allogeneic HCT care and monitoring, which was recently funded by PCORI (Patient Centered Outcomes Research Institute).
More information about this project can be found here: https://www.pcori.org/research-results/2024/reimagining-caregiving-together-engagement-address-caregiver-requirement-barriers.
CMS National Coverage Determination for MDS
By Carol Doleysh
The SCTOD is part of the US Health Resources and Services Administration (HRSA)-funded C.W. Bill Young Cell Transplantation Program that collects data on all allogeneic HCT performed in the US and on transplants done elsewhere using cellular products that originated in the US.
The CMS National Coverage Determination for allogeneic HCT for patients with MDS was effective March 6, 2024. CIBMTR is very appreciative of the collaborative efforts of participating transplant centers, physicians, and data managers in serving older Americans with MDS who have benefited from allogeneic HCT over the last 14 years.
Patients with Medicare insurance and meeting the criteria outlined by CMS receiving allogeneic HCT after March 6, 2024, should receive coverage based on the National Coverage Determination. There is no further role for Coverage with Evidence Development (CED) for this disease; new patients should no longer be asked to sign specific consent for the MDS CED study. Centers should continue to consent patients to participate in CIBMTR’s observational database protocol and continue to provide data for transplant recipients, including those who participated in the MDS CED.
Coverage decisions about patients with MDS who do not meet the criteria outlined by CMS in the National Coverage Determination will require a decision from local Medicare Administrative Contractors. Additional resources for billing for these services can be found here.
Publicly Available Datasets
 View a summary of the Publicly Available Datasets and the data dictionary containing the most commonly used variables.
View a summary of the Publicly Available Datasets and the data dictionary containing the most commonly used variables.
In accordance with the NIH Data Sharing Policy and NCI Cancer Moonshot Public Access and Data Sharing Policy, CIBMTR makes the final datasets from published studies publicly available on CIBMTR’s Research Datasets for Secondary Analysis webpage. These publication analysis datasets are freely available to the public for secondary analysis.
While providing these data, CIBMTR is committed to safeguarding the privacy of participants and protecting confidential and proprietary data. Upon accessing the datasets page on CIBMTR’s public website, the viewer is notified that the dataset was collected by CIBMTR, and CIBMTR’s supporters are listed. The webpage also clearly notes the terms and conditions of dataset usage.
NEW datasets are now available online.
Share Your Research in Plain Language
By Jennifer Motl
These new plain-language summaries of CIBMTR research may help your patients:
 |
Gilteritinib helps some people with leukemia; read more: |
 |
Guidelines needed for returning to school after transplant; read more: |
 |
Most children are alive and well after unrelated donor transplant for sickle cell disease; read more: |
|
|
1 year after transplant or cell therapy, patients’ social lives return; read more: |
 |
Transplant patients should get vaccinated for COVID-19 early; read more: |
Find more summaries on the Study Summaries for Patients webpage.
Our Supporters
CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; 75R60222C00008, 75R60222C00009, and 75R60222C00011 from the Health Resources and Services Administration (HRSA); and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research.
Additional federal support is provided by OT3HL147741, P01CA111412, R01CA100019, R01CA218285, R01CA231141, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01HL155741, R01HL171117, R21AG077024, U01AI069197, U01AI126612, U24HL157560, and UG1HL069254.
Support is also provided by Boston Children’s Hospital; Fred Hutchinson Cancer Center; Gateway for Cancer Research, Inc.; Jeff Gordon Children’s Foundation; Medical College of Wisconsin; NMDP; NYU Grossman School of Medicine; PBMTF; Rush University Medical Center; St. Baldricks’s Foundation; Stanford University; Stichting European Myeloma Network (EMN); University of Pittsburgh; and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc.; Amgen, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, inc.; Blueprint Medicines; Bristol Myers Squibb Co.; CareDx Inc.; CSL Behring; CytoSen Therapeutics, Inc.; DKMS; Editas Medicine; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gift of Life Biologics; Gift of Life Marrow Registry; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miller Pharmacal Group, Inc.; Miltenyi Biomedicine; Miltenyi Biotec, Inc.; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; OriGen BioMedical; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; PPD Development, LP; REGiMMUNE; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc.; Sobi, Inc.; Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, Department of the Navy, Department of Defense, Health Resources and Services Administration, or any other agency of the U.S. Government.

